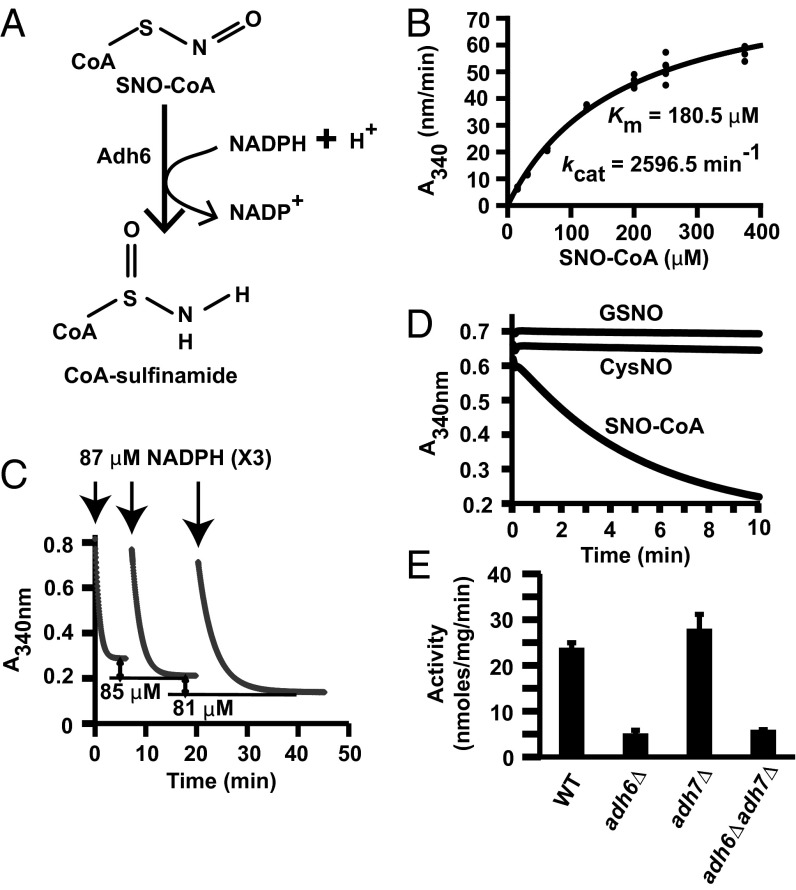
Fig. 2.
Characterization of the yeast SNO-CoA reductase, Adh6. (A) CoA-sulfinamide was identified by MS as the major stable product of SNO-CoA reduction by purified Adh6 (see Fig. S2 A, B, and D for product analysis). (B) Kinetic analysis of SNO-CoA reductase activity of purified Adh6. (C) Stoichiometry of NADPH:SNO-CoA in Adh6-catalyzed SNO-CoA reduction. Sequential additions of 87 μM NADPH to an excess of SNO-CoA led to consumption of 79–85 μM (mean 82 ± 3 μM; n = 6 additions) of SNO-CoA, demonstrating a stoichiometry of 1:1. Results shown are representative of two independent experiments. (D) Specificity of Adh6 for SNO-CoA. Purified Adh6 (Table S1 and Fig. 1D) (20 nM) was incubated with NADPH (100 µM) and SNO-CoA, GSNO, or CysNO (100 µM), and NADPH consumption was measured over time. (E) Adh6 is the principal source of NADPH-dependent SNO-CoA reductase activity. Activity was assayed in lysates from WT yeast and adh6Δ, adh7Δ, and adh6Δadh7Δ yeast. Data are presented as mean ± SD; n = 3.