Fig. 6.
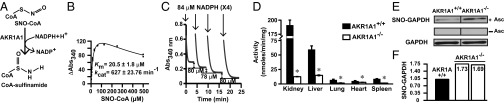
Characterization of the mammalian SNO-CoA reductase AKR1A1. (A) CoA-sulfinamide was identified by MS as the major stable product of SNO-CoA reduction by purified AKR1A1 (see Fig. S2 A, C, and D for product analysis). (B) Kinetic analysis of SNO-CoA reductase activity of purified AKR1A1. (C) Stoichiometry of NADPH:SNO-CoA in AKR1A1-catalyzed SNO-CoA reduction. Sequential additions of 84 μM NADPH to an excess of SNO-CoA led to consumption of 75–82 μM (mean 79 ± 3 μM; n = 8 additions) SNO-CoA, demonstrating a stoichiometry of 1:1. Results shown are representative of two independent experiments. (D) SNO-CoA reductase activity in AKR1A1 knockout animals. NADPH-dependent SNO-CoA reductase activity across various tissues from WT or AKR1A1−/− mice is shown. Extracts were incubated with 100 µM NADPH and 0 or 200 µM SNO-CoA. Values are from three WT (filled) and three AKR1A1−/− (open) mice. *P < 0.05 (Student t test). (E and F) Regulation of endogenous protein S-nitrosylation by AKR1A1. Analysis of S-nitrosylated GAPDH (SNO-GAPDH) in kidney extracts of AKR1A1+/+ and AKR1A1−/− mice is shown. Changes in SNO-GAPDH levels were determined by SNO-RAC coupled to Western blotting (E; n = 2) or to iTRAQ-based MS (F; n = 2).
