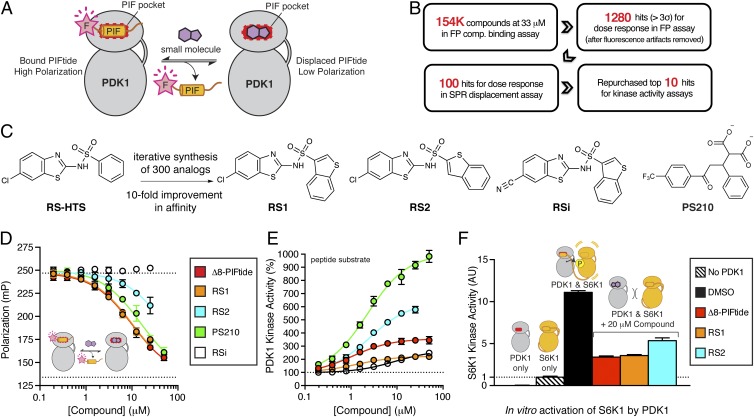
Fig. 1.
Discovery and optimization of diaryl sulfonamides as PIFtide mimics. (A) Schematic of a FP competitive binding assay developed to identify small-molecule mimics of the PIFtide. (B) Overview of the high-throughput screen and triage process. (C) Chemical optimization of the diaryl sulfonamide hit from HTS. RSi is an inactive analog used as a negative control. PS210 is a known PIF pocket ligand used as a positive control. (D) Dose–response curves for PIFtide, the RS compounds, and PS210 in the FP competitive binding assay. (E) Dose–response curves for PIFtide and the RS compounds in a radioactive kinase activity assay monitoring the phosphorylation of T308tide peptide substrate by PDK1. (F) Effect of PIFtide and the RS compounds on the in vitro activation of S6K1 by PDK1. After activation of S6K1 by PDK1 for 30 min, the kinase activity of S6K1 was determined by a radioactive kinase assay using the Crosstide substrate. The activity of S6K1 alone was used for normalization (dotted line). Error bars are ± SD (n = 3).