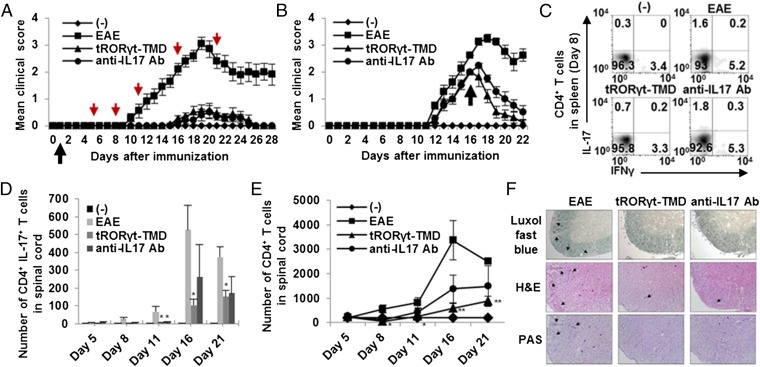
Fig. 4.
Preventive and therapeutic potential of tRORγt-TMD in the amelioration of EAE through inhibition of TH17 differentiation and function. (A and B) Clinical assessment of EAE mice injected with PBS (EAE), tRORγt-TMD (2 mg/kg), or anti-IL17 mAb (2 mg/kg) every other day from day 1 (preventive) (A) or day 16 (therapeutic) (B) after EAE induction. The black arrows indicate the point of the first injection of tRORγt-TMD, and the red arrows indicate the analyzed day (days 5, 8, 11, 16, and 21). The (-) control is normal mice. (C and D) Splenocytes and mononuclear cells from spinal cord were reactivated with PMA/ionomycin for 4 h, and then stained for anti-CD4 and intracellular-stained with anti–IL-17A/IFN-γ mAb followed by FACS analysis. Percentages of CD4+ IL-17+/IFN-γ+ T cells in spleen (C) and absolute number of CD4+ IL-17+ T cells in spinal cord (D) were measured. (E) Inhibition of CD4+ T-cell infiltration into the spinal cord by tRORγt-TMD during the amelioration of EAE. Mononuclear cells from spinal cord were prepared at different time point. The cells were then stained for CD4 and analyzed by FACS. The total numbers of CD4+ T-cell in spinal cord were measured. (F) Spinal cord sections obtained from each mouse at day 21 after EAE induction were analyzed for the extent of demyelination and inflammation. Data are representative of more than three experiments with 10 to 40 mice per group (A and B) or one experiment with at least three to five mice per group (C–F). Error bars denote SEM. *P < 0.05, **P < 0.01.