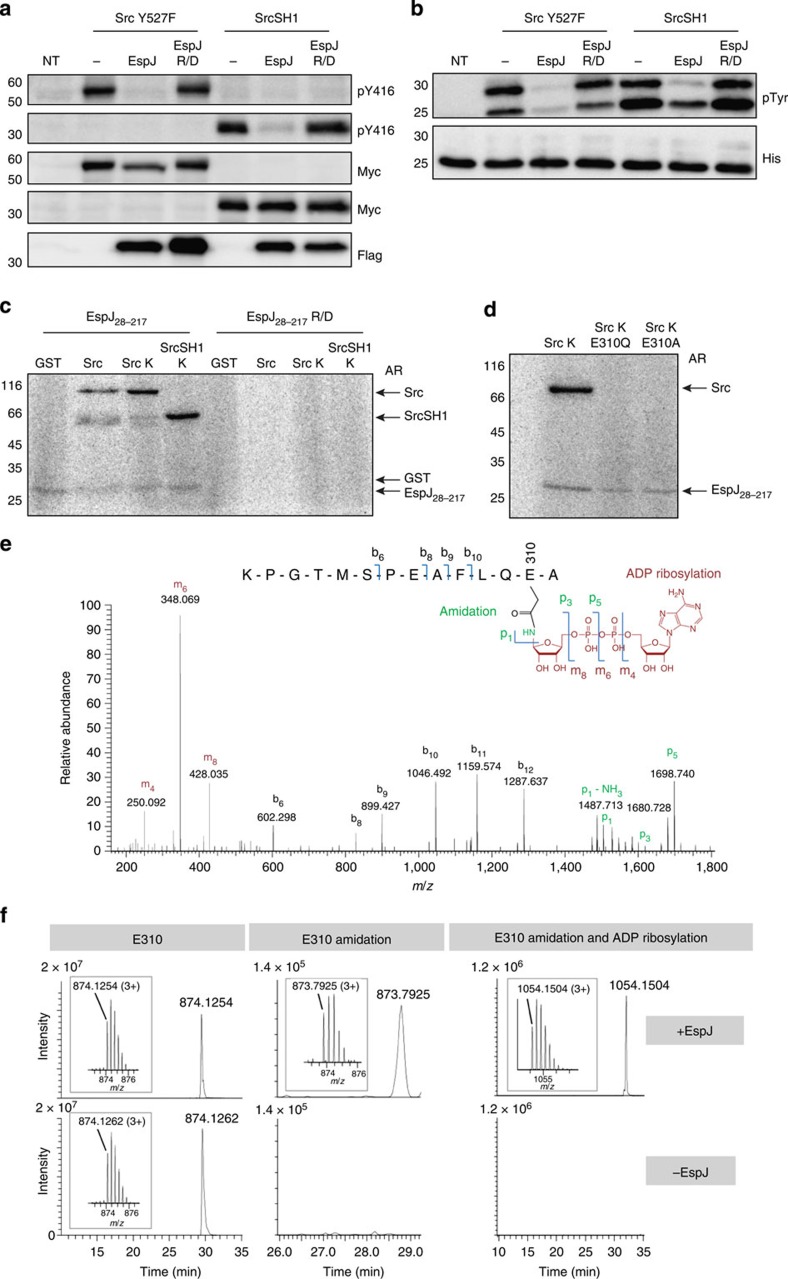
Figure 4. EspJ inhibits Src kinase activity by amidation and ADP ribosylation of Src E310.
(a) Swiss 3T3 cells co-expressing myc-tagged SrcY527F or SrcSH1 with Flag-tagged EspJ, or EspJ-R/D were analysed by anti-pY416, anti-Myc and anti-Flag antibodies. EspJ inhibited autophosphorylation (pY416) of both SrcY527F and SrcSH1. (b) Myc-tagged SrcY527F or SrcSH1 were immunoprecipitated from lysates of cells expressing EspJ or EspJ-R/D and incubated with His-tagged TirCEPEC. Analysis of TirCEPEC by immunobloting with anti-pTyr and anti-His antibodies indicated that SrcY527F and SrcSH1 were inactive when immunoprecipitated from cells co-expressing EspJ. Similar results were obtained in three independent experiments. Representative immunoblots are shown. The full immunoblots are shown in Supplementary Fig. 8. (c) Recombinant EspJ and EspJ EspJ-R/D were incubated with GST, GST-Src, GST-Src K295M (Src K) or GST-SrcSH1 K295M (SrcSH1 K) and 32P-labelled NAD+. Autoradiograph (AR) showing 32P-labelled Src and SrcSH1 in the presence of EspJ, but not EspJ-R/D. Corresponding commassie-stained PAGE gels are shown in Supplementary Fig. 9. Similar results were obtained in three independent experiments. (d) Recombinant EspJ was incubated with GST-Src K295M (Src K), GST-SrcK-E310Q or GST-SrcK-E310A and 32P-labelled NAD+. Autoradiograph showing 32P-labelling of only Src K. Similar results were obtained in two independent experiments. (e) HCD MS/MS spectrum of the precursor 1023.4071 (2+) corresponding to the amidated and ADP-ribosylated peptide with the sequence 298-KPGTMSPEAFLQEA-311 generated by digest of EspJ-incubated Src-K295M with elastase. Collision-induced fragmentation is observed at the backbone (b ion series) as well as in the ADP-ribose moiety (m and p ions). All p ions are shifted by −0.984 Da indicating the amidation (O to NH exchange) at the side chain of E310. (f) Src-K295M was incubated with or without addition of EspJ. Proteins were separated by SDS–PAGE, digested and analysed by nanoLC-MS/MS. Extracted ion chromatograms for the differentially modified tryptic peptide VAIMTLKPGTMSPEAFLQEAQVMK are shown: the unmodified sequence (E310), the amidated form of this peptide (E310 amidation) and the amidated and ADP-ribosylated form (E310 amidation and ADP ribosylation). The unmodified form of the tryptic peptide is detected in the presence and in the absence of EspJ, whereas the modified forms are only detectable when Src-K295M has been incubated with EspJ. Results were consistent in a repeat experiment using SrcSH1.