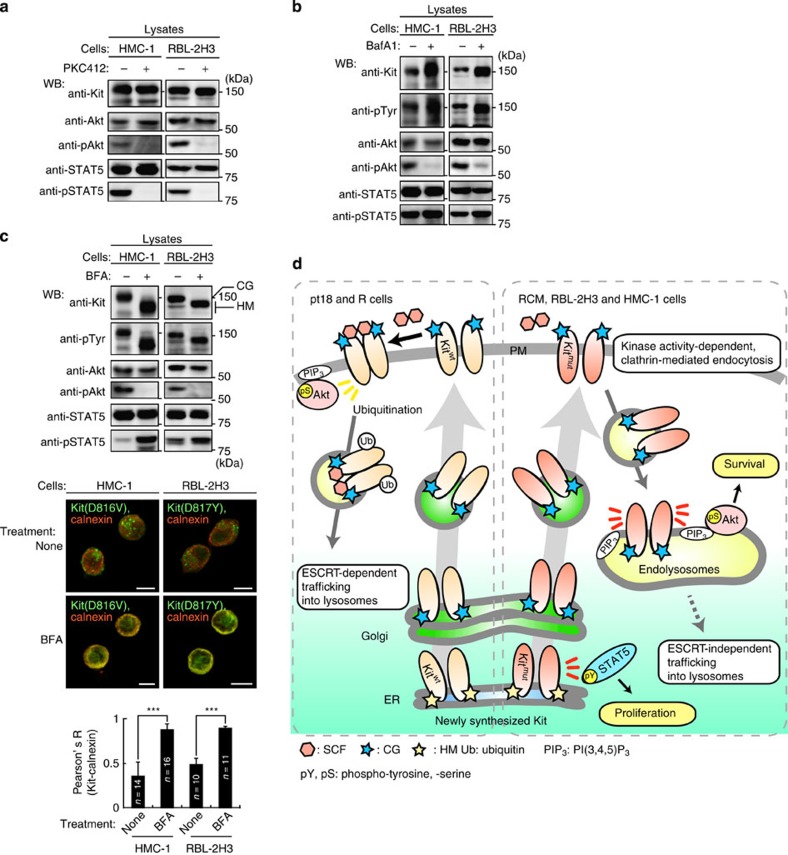
Figure 8. Oncogenic signalling by mutant Kit on endolysosomes and the ER in rat and human cells.
(a) Constitutive activation of Akt and STAT5 by mutant Kit in HMC-1 and RBL-2H3 cells. Immunoblots, HMC-1 (left) or RBL-2H3 cells (right) treated with 1 μM PKC412 for 4 or 12 h, respectively. (b) Immunoblots, HMC-1 or RBL-2H3 cells cultured with 100 nM BafA1 for 24 h. (c) (Top) Immunoblots, HMC-1 or RBL-2H3 cells cultured with 5 μM BFA for 16 h. CG, complex-glycosylated form; HM, high mannose form. (Middle) Cells stained with anti-Kit(green) and anti-calnexin (ER marker; red). Bars, 10 μm. (Bottom) The graph shows the correlation coefficient (Pearson’s R) between Kit and calnexin. Results are means±s.d. from 10 to 16 cells. ***P<0.001, Student’s t-test. (d) Trafficking and signalling from normal Kit (left) and mutant Kit (right). Normal Kit-left panel; newly synthesized Kit traffics from ER, through the Golgi apparatus to the PM. After binding to SCF, Kit activates downstream pathways such as PI3K-Akt mainly at the PM. Kit then becomes endocytosed and ubiquitinated, resulting in ESCRT-dependent incorporation into lysosomes and rapid degradation. Mutant Kit-right panel; soon after synthesis, immature Kit is localized on the ER and activates STAT5. It then traffics to the PM along the secretory pathway, similar to normal Kit. After mutant Kit reaches the PM, it immediately undergoes clathrin-mediated endocytosis in a kinase activity-dependent manner. Kit then accumulates to endolysosomes but is not fully ubiquitinated, so is resistant to degradation. Unlike normal Kit, mutant Kit-PI3K activates Akt specifically on endolysosomes. Mutant Kit does not require ESCRT for incorporation into lysosomes.