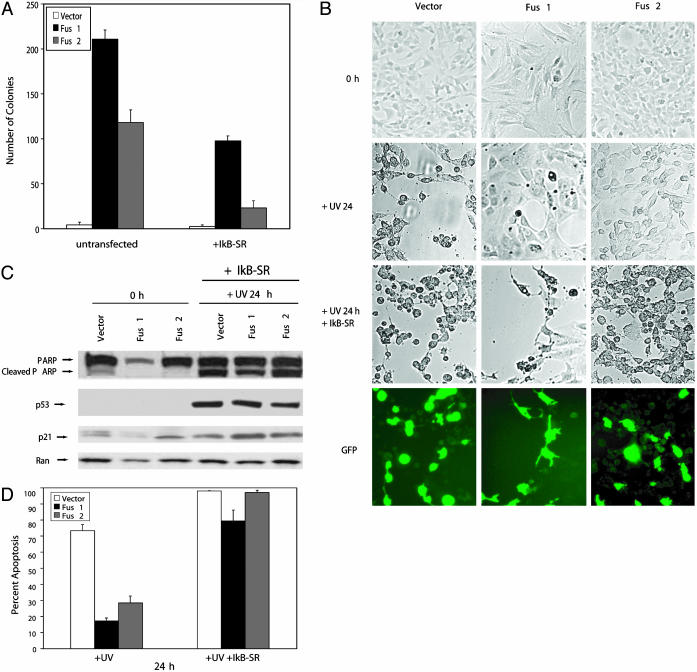
Fig. 5.
Transformation of NIH 3T3 cells and inhibition of p53-mediated apoptosis by Fus1 and Fus2 depend on NF-κB activation. (A) IκB-SR-transfected and untrasfected Fus1, Fus2, and vector cells were subjected to an anchorage-dependent growth assay in soft agar. Colony formation was reduced by 56% and 80% in Fus1 and Fus2 cells transfected with the IκB-SR compared with vector control. The data shown represent the mean ± SD obtained from three independent experiments performed in duplicate. (B) Treatment of Fus1, Fus2, and vector control cells with UVC after IκB-SR transient transfection resulted in similar amounts of PARP cleaved protein in Fus1-, Fus2-, and vector-expressing cells. p53 and p21 protein levels increased 24 h post UV damage in the transfected cells. This result is similar to the protein changes detected in untransfected Fus1-, Fus2-, and vector-expressing cells (Fig. 4B). Loading was controlled by anti-Ran monoclonal antibody. (C and D) Similar morphological changes indicative of cell death were detected in IκB-SR-transfected Fus1-, Fus2-, and vector-expressing cells 24 h post UV irradiation. In contrast, in untransfected cells, cell death in vector-expressing cells was significantly increased compared with FUS1- and FUS2-expressing cells. Apoptotic cells were visualized by DAPI (4′,6-diamidino-2-phenylindole) staining, and IκB-SR transfection efficiency was determined by GFP cotransfection. The data shown represent the mean ± SD obtained from three independent experiments performed in duplicate. Loading was controlled by anti-Ran monoclonal antibody.