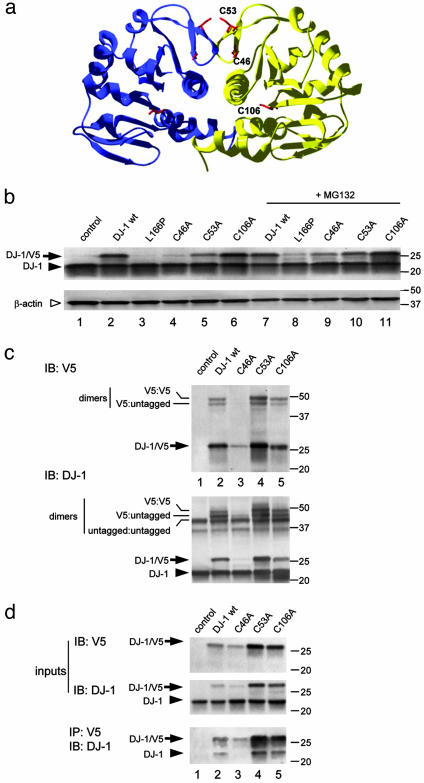
Fig. 1.
Generation of stable dimeric cysteine mutants of human DJ-1. (a) The crystal structure of human DJ-1 with cysteine residues highlighted. Two cysteine residues, C46 and C53, whose sidechains are shown in red, are present in a β-turn close to the dimer interface. A third cysteine, C106, is within the center of the dimer. (b) Transient transfections of V5-tagged cysteine mutants into M17 cells. Because some variants, notably C46A, were unstable, samples in lanes 7-11 were treated with 5 μM MG132 for 24 h. Western blots were probed with anti-DJ-1 (Upper) to visualize both transfected DJ-1 (arrows) and endogenous DJ-1 (arrowhead). The blot was reprobed with β-actin as a loading control (Lower, open arrowhead). Markers on the right of the blot are in kilodaltons. (c) To assess whether these variants form dimers, transfected cells were treated with the crosslinker disuccinimidyl suberate before extraction and Western blotting for V5 (Upper) or DJ-1 (Lower). Monomeric V5-tagged protein is indicated with an arrow, and endogenous DJ-1 in the cells indicated by an arrowhead. The V5-tagged proteins formed dimers with either endogenous or transfected DJ-1, indicated by lines on the left of the blot. Blots are representative of at least duplicate experiments. To confirm these results, we coimmunoprecipitated V5-tagged DJ-1 with endogenous protein (d). (Upper) Lysates of transfected cells probed with V5 or DJ-1 to demonstrate input levels of proteins. (Lower) Lysates immunoprecipitated withV5 antibody and blotted with DJ-1 antibody to show interaction of endogenous DJ-1 with the tagged proteins.