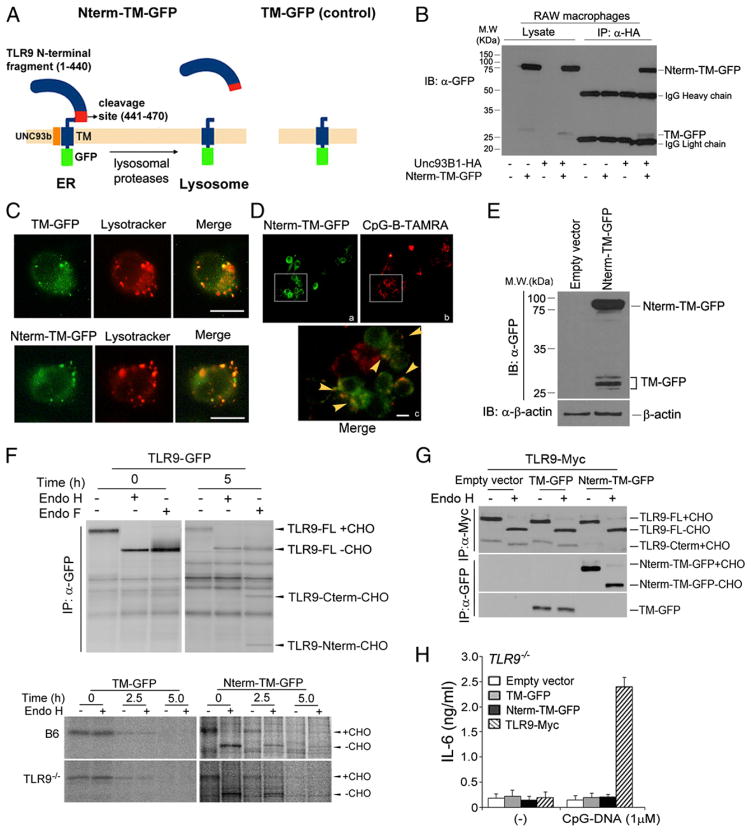
FIGURE 1.
Generation of lysosomal-targeted TLR9 N-terminal cleavage fragments. (A) Schematic showing generation of lysosomal-targeted TLR9 N-terminal chimeric constructs. Nterm-TM-GFP contains the N-terminal TLR9 proteolytic cleavage product, the cleavage sites (residues 441–470), TM, and GFP at the C terminus; TM-GFP encodes only the TLR9 TM and GFP. (B) RAW cells expressing TLR9 Nterm-TM-GFP in the presence or absence of Unc93B1-HA were lysed with 1% digitonin lysis buffer. Unc93B1 proteins were immunoprecipitated with the anti-HA Ab. Immunoprecipitated samples were resolved by SDS-PAGE and analyzed using anti-GFP Western blot analyses. (C) RAW cells expressing TM-GFP or TLR9 Nterm-TM-GFP were stimulated with 1 μM CpG-DNA for 1 h and washed. Cells were treated with LysoTracker and imaged using immunofluorescence assay. Scale bars, 5 μm. (D) RAW macrophages stably expressing TLR9 Nterm-TM-GFP were stimulated with 1 μM TAMRA-labeled CpG-DNA for 30 min, washed, and imaged after an additional 3-h incubation. Arrowheads indicate colocalization between Nterm-TM-GFP with CpG-B-TAMRA. Scale bars, 5 μm. (E) Lysates from RAW macrophages stably expressing Nterm-TM-GFP were analyzed by Western blot analyses using anti-GFP or anti–β-actin Ab. (F, upper panels) Wild-type TLR9-GFP–expressing macrophages were labeled with [35S]methionine/cysteine and chased for 5 h. Cell lysates were immunoprecipitated with the anti-Myc Ab, and immunoprecipitates were treated with Endo H or Endo F. (F, bottom panels) B6 or TLR9−/− immortalized macrophages stably expressing TM-GFP or Nterm-TM-GFP were labeled with [35S]methionine/cysteine for 30 min and chased for the indicated times. Each chimeric protein was recovered using the anti-GFP Ab, followed by treatment with Endo H. (G) Nterm-TM-GFP does not affect trafficking of wild-type TLR9. RAW cells expressing empty vector, TM-GFP, or TLR9 Nterm-TM-GFP in the presence of full-length TLR9-Myc were lysed with 1% NP-40 lysis buffer. Lysates were immunoprecipitated with the anti-Myc or anti-GFP Ab. Immunoprecipitated proteins were resolved by SDS-PAGE and analyzed using anti-Myc or anti-GFP Western blot analyses. (H) The N-terminal TLR9 product does not activate TLR9 signaling. ELISA showing IL-6 production by TLR9−/− immortalized macrophages stably expressing empty vector, TM-GFP, Nterm-TM-GFP, or wild-type TLR9, stimulated for 12 h with CpG-DNA (1 μM). Data are representative of at least three independent experiments (average ± SD).