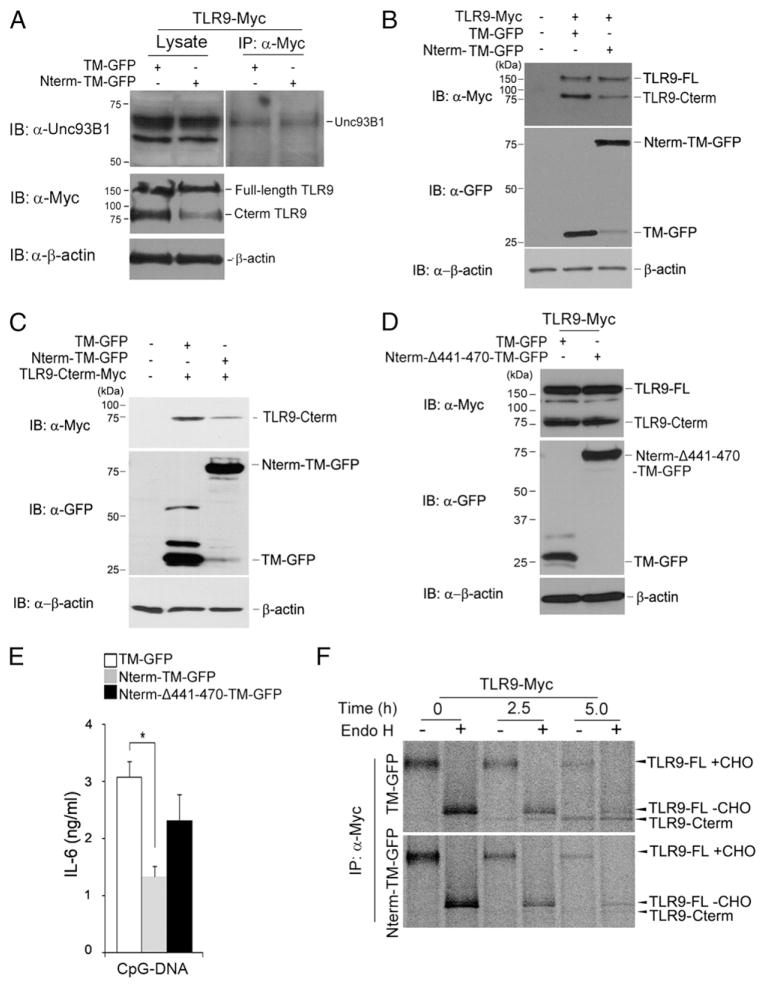
FIGURE 3.
The N-terminal cleavage product degrades C-terminal active receptor. (A) RAW macrophages expressing either TM-GFP or TLR9 Nterm-TM-GFP were lysed with 1% digitonin lysis buffer. TLR9-Myc proteins were immunoprecipitated with the anti-Myc Ab. Immunoprecipitated samples were resolved by SDS-PAGE and analyzed by Western blot analyses using the Unc93B1, Myc, or β-actin Ab. (B) TLR9 stability in RAW macrophages expressing either TM-GFP or Nterm-TM-GFP was monitored by immunoblotting with anti-Myc, anti-GFP, or anti–β-actin. Full-length TLR9 was tagged at the C terminus with Myc. TLR9-FL, full-length TLR9; TLR9 Cterm, C-terminal fragment of TLR9. (C) Immunoblot analyses of the recombinant Myc-tagged C-terminal TLR9 fragment (residues 471–1032) in RAW macrophages expressing either TM-GFP or Nterm-TM-GFP. Lysates were immunoblotted with anti-Myc, anti-GFP, or anti–β-actin. (D) Immunoblot analyses of the recombinant Myc-tagged C-terminal TLR9 fragment in RAW macrophages expressing either TM-GFP or Nterm-Δ441-470-TM-GFP. (E) IL-6 production by macrophages stably expressing TM-GFP or Nterm-Δ441-470-TM-GFP exposed for 12 h to CpG-DNA (1 μM). *p < 0.01 (Student t test). (F) Wild-type TLR9-Myc–expressing macrophages stably expressing either TM-GFP or Nterm-TM-GFP were labeled with [35S]methionine/cysteine for 1 h and chased for the indicated times. Cell lysates were immunoprecipitated with the anti-Myc Ab, and immunoprecipitates were treated with Endo H. Data are representative of three independent experiments. Data are representative of at least three independent experiments (average ± SD).