Fig 6.
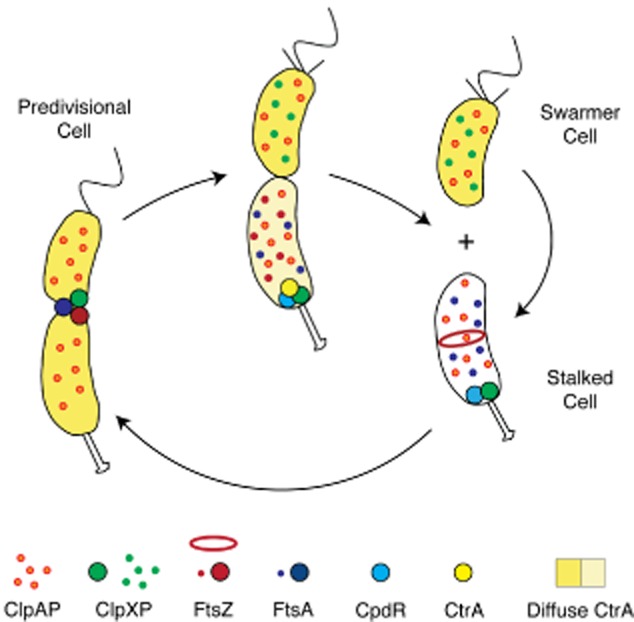
Schematic of the dynamic deployment of the ClpXP and ClpAP proteases and the FtsZ and FtsA divisome components during Caulobacter cell cycle progression. ClpXP and ClpAP are diffuse in the swarmer cell. Upon transition from a swarmer to stalked cell, the CpdR single domain response regulator localizes to the incipient stalked pole where it functions to recruit ClpXP to the cell pole. There, ClpXP degrades the polar flagellar basal body, components of the chemotaxis complex, and the CtrA inhibitor of DNA replication (Iniesta et al., 2006). As the stalked cell transitions to a predivisional cell, CpdR is degraded and ClpXP is freed from the cell pole (Iniesta and Shapiro, 2008). Near the end of the division process, ClpXP is recruited to the FtsZ ring (McGrath et al., 2006), while ClpAP remains diffuse throughout the cell cycle. Following cell division, ClpAP degrades FtsA while both ClpXP and ClpAP degrade FtsZ in the swarmer cell, but not in the daughter stalked cell.
