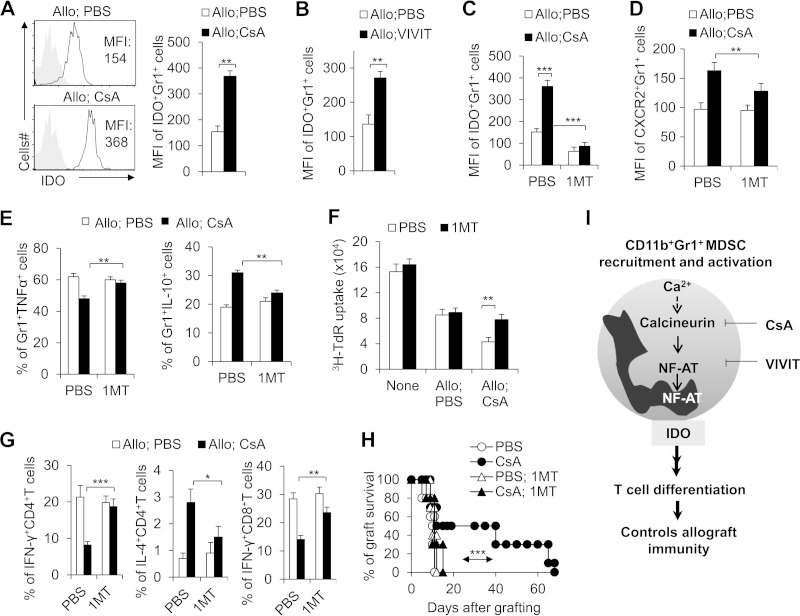
FIG 8.
IDO is required for the functional activities of CsA-induced CD11b+ Gr1+ MDSCs in prolonging allograft survival. (A) Significantly higher IDO expression in splenic CD11b+ Gr1+ cells from CsA-treated allograft recipient mice compared with PBS-treated control mice. MFI, mean fluorescence intensity. (B) Blocking NFAT activity significantly increased the IDO MFI expression in CD11b+ Gr1+ MDSCs. (C to F) Blocking IDO expression with 1MT (400 mg/kg, gavage daily) significantly diminished the MFI expression of IDO (C) and CXCR2 (D) and recovered the production of TNF-α and IL-10 (E) in CD11b+ Gr1+ MDSCs from CsA-treated allograft recipient mice compared with PBS-treated control group and diminished the suppressive activity of CsA-treated CD11b+ Gr1+ MDSCs in vitro (F). (G) DTH responses were examined. Approximately 2 weeks after OTII or OTI mice had been immunized with OVA (1 mg/ml), OTII CD4+ T cells or OTI CD8+ T cells in the spleen and lymph nodes were shown to be enriched using the negative selecting MACS kit for CD4+T cells or CD8+ T cells. CD11b+ Gr1+ cells (CD8− cells; 5 × 105 cells each) isolated from the spleens of C57BL/6 mice from the indicated treatment allograft recipient mice were used as APCs for OVA. Responder cells (OTII CD4+ T cells or OTI CD8+ T cells) and CD11b+ Gr1+ cells (5 × 105 cells each) with OVA were injected intradermally into the lateral part of the ears of naive CD45.1+ C57BL/6 mice. At 48 h after the challenge, the dLN lymphocytes were isolated, and IFN-γ and/or IL-4 expression in the donor (CD45.2+) CD4+ T cells or CD8+ T cells was analyzed by intracellular staining, and the data were summarized. (H) The indicated allograft recipient mice were treated with 1MT (400 mg/kg, gavage daily) or without IMT. The mice were sacrificed 8 days later, and 1 × 106 CD11b+ Gr1+ cells were sorted from the spleen and transferred into the same C57BL/6 recipient mice pretreated with an anti-Gr1 MAb (0.5 mg) 1 day earlier. Allograft recipient mice underwent skin transplantation 12 h after cell transfer, and the graft survival curve was plotted (n = 10). (I) Model summarizing the role of calcineurin-NFAT axis in controlling allograft immunity in CD11b+ Gr1+ MDSCs. Blocking the calcineurin-NFAT axis with CsA and VIVIT promotes CD11b+ Gr1+ MDSC activity, recruitment, and IDO expression. IDO is critical for the MDSC suppressive activities and directing reciprocal differentiation in CD4+ T cells between TH1 and TH2 or differentiation of CD8+ T cells in prolonging allograft survival. The data in this figure are representative of three (A, F, and G) or two (B to E and H) independent experiments (for panels A to G, n = 3 to 5). Values that are statistically significantly different are indicated by bars (or an arrow) and asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.