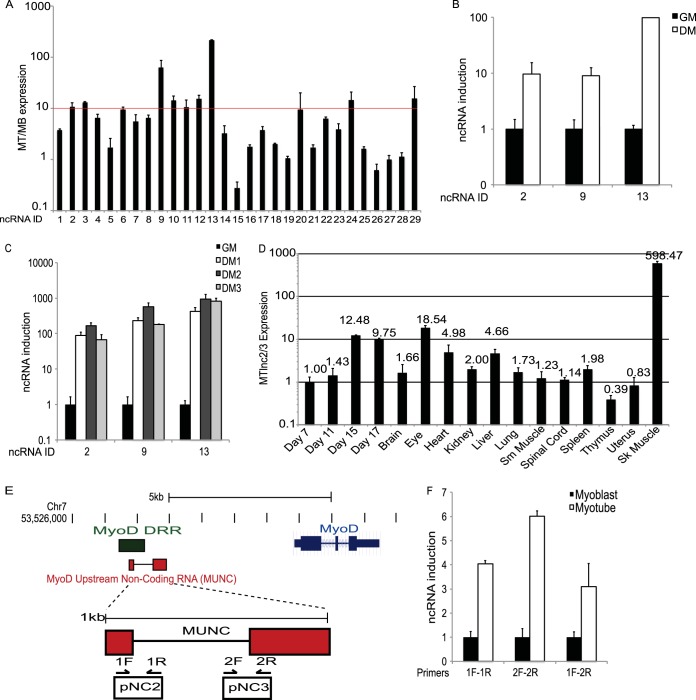
FIG 2.
Predicted muscle-specific noncoding RNAs are upregulated in myotubes. (A) qPCR confirmation of predicted noncoding RNAs induced in myotubes versus myoblasts. Nine predicted RNA-Seq fragments at seven independent genomic loci were >10-fold induced in myotubes (MT) versus levels in myoblasts (MB). (B) qPCR analysis of MyoD transfected transdifferentiating 10T1/2 fibroblasts for several predicted myogenically regulated lncRNAs when cells were transferred to low-serum differentiation medium (DM) versus growth medium (GM). (C) RT-qPCR of lncRNAs from screen in differentiating C2C12 myoblasts on the indicated days after the switch to low-serum differentiation medium. (D) RT-qPCR of lncRNAs 2 and 3 (or MUNC) from mouse embryos (embryonic days 7, 11, 15, and 17) and murine tissues showing high expression in skeletal muscle. (E) Schematic of MUNC genomic region upstream of the MyoD1 locus. MUNC overlaps the previously characterized distal regulatory region (DRR) enhancer and putative noncoding transcripts 2 and 3 (pNC2 and pNC3). (F) RT-qPCR analysis of MUNC expression in primary myoblasts and myotubes. MUNC 1F-1R and 2F-2R primers measure the primary unspliced MUNC while the 1F-2R primers measure spliced MUNC (Fig. 3E). Data represent means ± standard errors of the means (n = 3).