Figure 4.
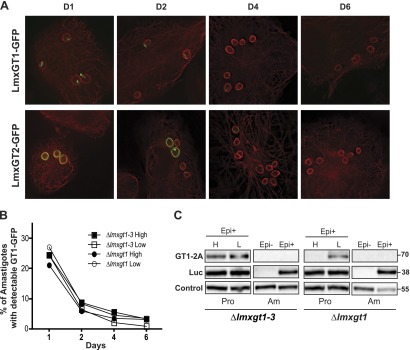
Expression of LmxGT1-GFP and LmxGT2-GFP following infection of macrophages. A) THP-1 macrophages were infected with ∆lmxgt1-3 null mutants expressing each GFP fusion protein, and deconvolution fluorescence images were recorded for cells at days 1, 2, 4, and 6 postinfection. Green represents GFP, and red represents fluorescence from an anti-α-tubulin antibody. B) The percentage of amastigotes with detectable GFP signal was plotted vs. days postinfection for ∆lmxgt1-3 and ∆lmxgt1 null mutants expressing LmxGT1-GFP from an episomal expression vector in high and low glucose. Typically, 80–-150 amastigotes were counted per sample. C) Loss of LmxGT1-TaV2A signal when parasites transform from promastigotes to amastigotes. Lines encoding the LmxGT1-TaV2A-Luc ORF on an episomal vector were prepared in the ∆lmxgt1-3 and ∆lmxgt1 null mutant backgrounds. Expression of GT1-2A and Luc from the cotranslationally cleaved translation product was monitored by Western blot by use of anti-TaV2A and anti-Luc antibodies. For amastigotes (Am), lysates were prepared from parasites without the episome (Epi–) as a negative control and from parasites encompassing the LmxGT1-TaV2A-Luc-encoding episome (Epi+). For promastigotes (Pro), lysates were prepared from early logarithmic-phase parasites (day 2 of growth) of the episome-carrying (Epi+) line, cultured in the presence of high glucose (H) or low glucose (L). In this experiment, the LmxGT1-TaV2A protein apparently began to down-regulate in the high glucose-cultured promastigotes in the ∆lmxgt1 line (fifth lane from the left) more rapidly than it did in the ∆lmxgt1-3 line (first lane from the left). The band marked Control refers to a cross-reacting signal used as a loading control.
