Figure 3. NADPH, putative sterol binding pockets and homology modeling with steroid 5β-reductase.
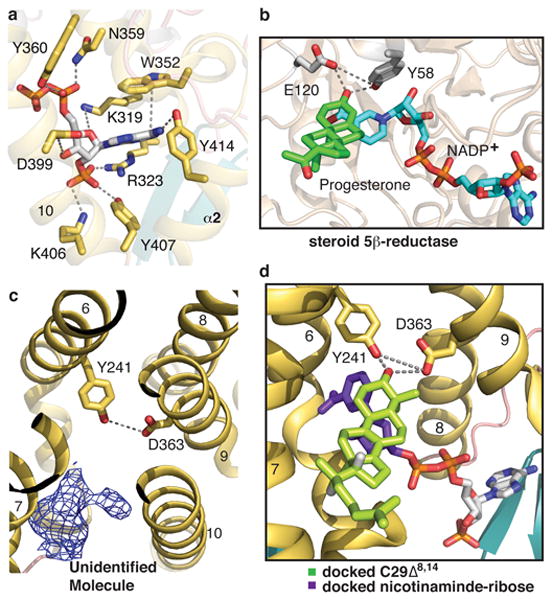
a, Close-up view of the NADPH binding pocket. NADPH is shown in stick representation with the phosphates in red. The interactions between NADPH and maSR1 are indicated by dash lines. b, Close-up view of the active pockets of steroid 5β-reductase (PDB 3COT). Tyr58 and Glu120 clamp the β3 carbonyl oxygen of progesterone. c, The putative sterol binding site is accessible from the lipid bilayer. An unidentified ligand density is shown with mFo-DFc map (blue mesh) at σ level of 2.5. d, Modeling of maSR1 putative sterol binding pocket. Based on the active sites of steroid 5β-reductase, the missing ribose-nicotinamide moiety (purple) of NADPH was docked into the NADPH pocket and C29Δ8,14 (light green) modeled into the pocket of the maSR1 structure.
