Abstract
In Europe, 6,000 young people die of cancer yearly, the commonest disease causing death beyond the age of 1 year. In addition, 300,000–500,000 European citizens are survivors of a childhood cancer and up to 30% of them have severe long-term sequelae of their treatment. Increasing both cure and quality of cure are the two goals of the European paediatric haematology/oncology community. SIOPE coordinates and facilitates research, care and training which are implemented by the 18 European study groups and 23 national paediatric haematology/oncology societies. SIOPE is the European branch of the International Society of Paediatric Oncology and one of the six founding members of the European Cancer Organisation. SIOPE is preparing its strategic agenda to assure long-term sustainability of clinical and translational research in paediatric malignancies over the next 15 years. SIOPE tackles the issues of equal access to standard care and research across Europe and improvement of long term follow up. SIOPE defined a comprehensive syllabus for training European specialists. A strong partnership with parent, patient and survivor organisations is being developed to successfully achieve the goals of this patient-centred agenda. SIOPE is advocating in the field of EU policies, such as the Clinical Trials Regulation and the Paediatric Medicine Regulation, to warrant that the voice of young people is heard and their needs adequately addressed. SIOPE and the European community are entirely committed to the global agenda against childhood cancers to overcome the challenges to increasing both cure and quality of cure of young people with cancer. Pediatr Blood Cancer 2014;61:1551–1557.
Keywords: cancer, care, education, oncopolicy, research
CANCER IN YOUNG PEOPLE IN EUROPE
Every year in Europe 15,000 children aged 0–14 years and 20,000 teenagers and young adults aged 15–24 years are diagnosed with cancer [1]. Overall survival at 5 years continuously improved from 76.1% in 1999–2001 to 79.1% in 2005–2007 [2]. However, 6,000 young people in Europe still die of cancer each year despite best available treatments. No progress has been made for malignancies with the worst prognosis (brain tumours, neuroblastoma, sarcomas and acute myeloid leukaemia). Across Europe there are still major disparities in 5-year survival, for example Eastern Europe reports 10–20% lower survival rates [2]. Cancer remains the commonest disease causing death beyond the age of 1 year in Europe. It is estimated that 300,000–500,000 European citizens are survivors of a childhood cancer: 60% of them have at least one chronic health problem and 30% have severe long-term sequelae [3]. Increasing both cure rate and quality-of-cure (defined by presence and intensity of treatment complications in a long-term survivor) for young people with cancer are the two goals for the next decade. The types of cancer occurring in this age group in Europe are similar to those observed in the rest of the world [4].
SIOP-EUROPE, THE PAN-EUROPEAN SOCIETY FOR PAEDIATRIC HAEMATOLOGY/ONCOLOGY
SIOPE is the European branch of the International Society of Paediatric Oncology (SIOP), and participates actively in the global agenda, fighting childhood cancer [5]. SIOPE is also one of the six founding members of the European CanCer Organisation (ECCO) and thus participates actively in the European multidisciplinary oncology agenda with cancer societies of medical oncologists, radiation oncologists, surgical oncologists, oncology nurses and scientists as well as with the broader parents and patients advocate community [6]. The clinical challenges of rare conditions are shared by all children with cancer as well as many adult patients with rare variants, now increasingly determined by molecular sub-typing as part of the personalised medicine agenda [7]. The recently emerged focus upon teenagers and young adults (TYA) at the interface between paediatric and adult medicine is a fruitful area for research collaboration and shared clinical service development [8]. SIOPE will further develop collaboration with the European Society of Medical oncology to better tackle the TYA issues. It is hoped that improvements in both survival and quality-of-survival will come from dedicated multi-professional teams, with experts from paediatric and adult cancer backgrounds collaborating. SIOPE sees itself as an important stakeholder in both the Global Paediatric Oncology and the European Oncology agendas.
SIOPE coordinates and facilitates European activities in research, care and training. There are 23 National Paediatric Haematology Oncology Societies or groups (NAPHOS; Fig. 1; Table 1) and 18 European study groups (Table 2) running the European research and care agenda in more than 60 different paediatric malignancies and cross-cutting areas such as early drug development, extremely rare cancers, teenagers and young adults and long-term follow-up. Representatives from both NAPHOS and from European Study Groups have joined together to form the SIOPE European Clinical Research Council (ECRC). The ECRC represents the common, harmonised voice for advocacy and lobbying at the European level on paediatric and adolescent cancer clinical and research activities.
Fig 1.
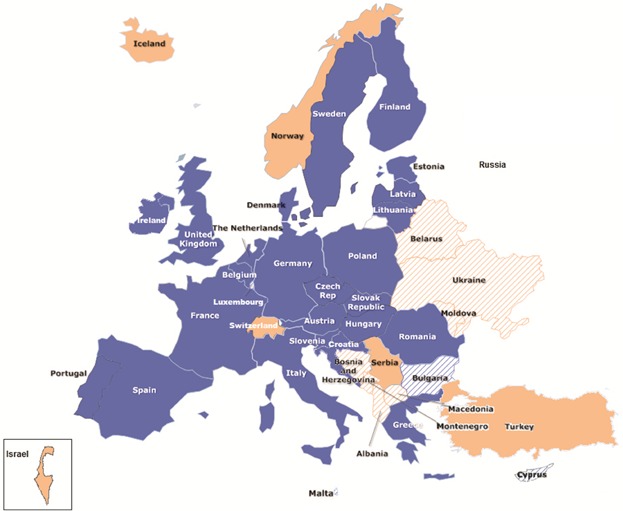
Map of paediatric haematology oncology in Europe. The figure shows member states from the European Union (blue) and out of the European Union (pink), as well as member states with (plain colour) or without (dashed) a National Paediatric Haematology Oncology Society.
TABLE I.
National Paediatric Haematology Oncology Societies or Groups (NAPHOS) in Europe
| Country | Name | Acronym |
|---|---|---|
| Austria | Austrian Group for Paediatric Haemato-Oncology | AGPHO |
| Belgium | Belgian Society of Paediatric Haematology Oncology | BSPHO |
| Czech Republic | Czech Working Group for Paediatric Oncology | CPH |
| Denmark, Finland, Iceland, Norway, Sweden | Nordic Society of Paediatric Haematology & Oncology | NOPHO |
| Estonia | Baltic Society of Paediatric Haemato-Oncology: Estonia | BSPHO |
| France | Société Française de lutte contre les Cancers et leucémies de l'Enfant et l'adolescent | SFCE |
| Germany | Gesellschaft für Pädiatrische Onkologie und Hämatologie | GPOH |
| Greece | Hellenic Society of Paediatric Haematology-Oncology | |
| Hungary | Hungarian Paediatric Oncology Network | HPOG |
| Italy | Associazione Italiana Ematologia Oncologia Pediatrica | AIEOP |
| Latvia | Latvian Society of Paediatric Oncology | |
| Lithuania | Lithuanian Society of Paediatric Oncology and Haematology | LVOHD |
| Netherlands | Stichting Kinderoncologie Nederland | SKION |
| Poland | Polish Society of Paediatric Oncology and Haematology | |
| Portugal | Sociedade de Hematologia e Oncologia Pediatrica | SHOP |
| Romania | Romanian Society of Paediatric Haematology/Oncology | |
| Serbia | Serbian Society of Haematology and Oncology | |
| Slovak Republic | Slovak Paediatric Association-Section of Paediatric Haemato-Oncology | |
| Slovenia | Slovenian Society of Paediatric Haematology and Oncology | |
| Spain | Sociedad Española de Hematología y Oncología Pediátricas | SEHOP |
| Switzerland | Schweizerischen Pädiatrischen Onkologie Gruppe | SPOG |
| Turkey | Turkish Paediatric Oncology Group | TPOG |
| United Kingdom and Ireland | Children's Cancer Leukaemia Group | CCLG |
TABLE II.
European and Europe-Centred International Study Groups in Paediatric Haematology/Oncology
| Acronym | Name | Area | Website |
|---|---|---|---|
| CWS | Cooperative Weichteilsarkom Studiengruppe or Cooperative Soft Tissue Sarcoma Study Group | Soft tissue sarcomas | |
| EBMT-PDWP | European Group for bone marrow and stem cell transplantation: Paediatric Diseases Working Party | Transplantation | www.ebmt.org |
| EHL | European Hodgkin Consortium | Hodgkin | |
| EICNHL | European Inter-group cooperation on childhood and adolescent Non Hodgkin Lymphoma | Non-Hodgkin Lymphoma | |
| EpSSG | European Paediatric Soft Tissue Sarcoma Study Group | Soft tissue sarcomas | www.epssg.cineca.org |
| EURAMOS | European and American Osteosarcoma Study Group | Osteosarcoma | www.euramos.org |
| EURO-EWING | European Inter-group cooperation on Ewing tumours | Ewing tumours | |
| EWOG-MDS | European Working Group of myelodysplasic syndrom and juvenile myelomonocytic leukaemia in Childhood | Myelodysplasia | www.ewog-mds.org |
| EXPeRT | European Cooperative Study group on Paediatric Rare Tumours | Extremely Rare cancers | |
| GCT-group | Germ Cell Tumours | Germ cell tumours | |
| Histiocyte Society | Histiocyte Society | Histocytosis | www.histiocytesociety.org |
| I-BFM | International BFM Study Group | Leukaemia's | www.bfm-international.org |
| ITCC | European Consortium for Innovative Therapies for Children with Cancer | Early drug trials | www.itcc-consortium.org |
| PanCare | Pan-European Network for Care of Survivors after Childhood and Adolescent Cancer | Long-term follow-up and Survivorship issues | www.pancare.eu |
| SIOP RTSG | SIOPE Wilms Tumour Study Group | Wilms tumour | www.siop-rtsg.eu |
| SIOPE-Brain | SIOPE Brain tumour group | Brain tumours | |
| SIOPEL | SIOPE Childhood Liver Tumours Strategy Group | Liver tumours | www.siopel.org |
| SIOPEN | SIOPE Neuroblastoma Group | Neuroblastoma | www.siopen-r-net.org |
During the last 10 years, SIOPE has increasingly represented the voice of all those involved in paediatric and TYA cancer at the EU political level. Proactive in its approach, the SIOPE Office in Brussels with the SIOPE Board ensures that our community's concerns are heard and we strongly advocate for them to be taken into account in EU policies, regulations and legal initiatives. There is still the need for policy makers and regulators to act on our proposals and new policies are required to address adequately the global burden of childhood cancers [9].
ASSURING LONG-TERM SUSTAINABILITY OF CLINICAL AND TRANSLATIONAL RESEARCH IN PAEDIATRIC MALIGNANCIES IN EUROPE
In 2008, SIOPE in collaboration with the European Study Groups identified pitfalls and hurdles associated with running clinical and translational research in order to define a strategy for the next decades. Despite a long history of networking that generated successful advances in cure rate, clinical research was still fragmented in Europe and most groups and institutions faced major difficulties to run investigator-driven clinical trials under the EU Clinical Trials Directive. There was limited access to new oncology drugs developed by pharmaceutical companies, despite the introduction of the 2007 EU Paediatric Medicines Regulation. The EU has funded several translational research projects in paediatric cancers such as the KidsCancerKinome and European Embyronal Tumour (EET)-pipeline projects but more needed to be done. Integration between biology and clinical research remains inadequate, at a time when personalised cancer medicine is emerging. There is such a great disparity in access to the best standard of care or trials of new therapies within research-based care across Europe that impacts on survival outcomes for the children [2]. Collaboration with parent organisations and survivors needs to be strengthened and a lack of sustained and sufficient funding in the context of the global economic crisis will further jeopardise delivery of the research agenda.
The EU recognised the need for a “virtual institute” for childhood and adolescent cancer. In 2011, the EU offered SIOPE and 32 associated institutions and organisations the opportunity to create the EU-funded European Network for Cancer research in Children and Adolescents (ENCCA) [10]. This 4-year network of excellence aims to structure a sustainable clinical and translational research agenda in Europe over the next 15 years and build the necessary tools and platforms to achieve further improvements in both cure rate and quality-of-cure (Table 3). Just over 2 years into the program, ENCCA is progressing efficiently and is preparing the long-term sustainable project for paediatric haematology/oncology in Europe.
TABLE III.
The 10 Goals and Output of the European Network for Cancer Research in Children and Adolescents (ENCCA)
| Goal | Output | |
|---|---|---|
| 1 | Create a sustainable “European Virtual Institute” for clinical and translational research in childhood and adolescent cancers | Run the global European strategic plan |
| Facilitation of the implementation of investigator-driven clinical trials | ||
| Rapid translation of new knowledge into patient care | ||
| 2 | Define the European Strategy to increase both cure and quality of cure at the Horizon 2020 | The seven medical and scientific objectives and road-map till 2020 |
| The means and needs to run this strategic plan | ||
| 3 | Integrate all relevant stakeholders and enhance collaboration | Ways to commit all stakeholders (clinicians, health professionals, biologists, researchers, imaging developers, epidemiologists, statisticians, drug developers, IT partners, parents and patients, industry, ethical and regulatory authorities) |
| 4 | Reduce knowledge fragmentation and improve communication | Strengthened integration of leukaemia and tumour groups |
| Increased critical mass of expertise and capacity to speed up clinical research integrated into care | ||
| 5 | Improve therapeutic strategies by enabling better access to innovative therapies, knowledge sharing and innovative technology, | A biology-based therapeutic strategy for each paediatric malignancy |
| Shared and mutualised clinico-biological databases | ||
| Prioritisation of new drug development within the European Paediatric Medicine Regulation | ||
| 6 | Improve the quality of life of children and adolescents with cancer with particular emphasis on long term treatments side effects | A “Survivorship passport” available for each patient cured of a paediatric malignancy |
| Facilitation of adequate risk-based advice, follow-up and care | ||
| 7 | Improve access to care and research for teenagers and young adults (TYA) | Sharing practice and promoting service development, interdisciplinary support and specific guidance for care of TYA |
| Strengthened collaboration with adult oncology | ||
| Creation of European TYA Steering groups with all stakeholders and leading health professionals | ||
| 8 | Promote Innovative methodologies and designs for clinical trials | Further address the rarity of patients and conditions to speed up the evaluation of new drugs and new biomarker-driven therapeutic strategies |
| 9 | Organize a comprehensive education and training programme | For health professionals (clinicians, nurses and all professionals in multidisciplinary teams), to facilitate the implementation of standards of care across Europe as well as clinical research |
| For parents and patients, to increase their awareness on clinical research | ||
| 10 | Propose common ethical definitions and adequate monitoring of ethical issues | Identified, documented and classified ethical issues encountered in paediatric oncology research |
| Guidelines |
European Research is funded on a per project basis through national and European grants as well as charities. Industry funds less than 5% of projects. For the first time ever, funding was made available through ENCCA (12 M € for 4 years) to structure clinical and translational research in Europe. One of the key challenges for long-term sustainability will be to secure this structure funding for integration, coordination and implementation of the research EU agenda in paediatric haematology/oncology.
SIOPE is linking with other appropriate pan-European initiatives such as the European Partnership for Actions Against Cancer (EPAAC) for cancer outcomes research and advocacy [11], and the EUROCANPLATFORM network for translational research and precision medicine [12].
EQUAL ACCESS TO STANDARD OF CARE AND RESEARCH ACROSS EUROPE
Equal access to the best standard of care across Europe is a major goal for SIOPE. Acknowledging that health is dealt with at the national level (a Member State competency) and is not a European prerogative, SIOPE and the International Confederation of Childhood Cancer Parent Organisations (ICCCPO), considered that guidelines outlining the minimum requirements for delivery of standard of care would be a useful advocacy tool. Such guidelines could help health professionals and parents' and patients' groups to address inequalities by convincing their national health authorities to optimise their efforts to reach and exceed 80% overall cure rates. Through EPAAC [11], SIOPE worked with all health professionals, including paediatric oncologists, nurses, physiotherapists and psycho-social care workers as well as patient advocates, to design and validate the European standards of care for Children with cancer which are now available on the SIOPE website in 16 languages [13].
Treating children with cancer is complex and needs a multidisciplinary specialised team. The European hub-and-spoke model is already being set up in several countries, such as the United Kingdom and France. In this model, a limited number of specialist centres (the hubs) are responsible for accurate diagnosis, risk-stratified treatment decision and complex treatments [3]. They liaise with local centres (the spokes) to share the patient care, providing less complex treatments such as simple chemotherapy and components of supportive care along with careful monitoring closer to patient's home. A limited number of hub centres are also resourced to deliver even more specialised treatments requiring expert teams in for example: complex surgery, high precision radiation therapy in vulnerable locations, high-dose chemotherapy with haematopoietic stem cell support, access to innovative drugs in early phase trials, MIBG therapy for high-risk neuroblastoma and treatment for extremely rare cancers.
The number of centres with this higher level of expertise in Europe is limited and referral of patients to these centres in other regions and Member States needs to be coordinated. To support patient treatment across borders, the EU Cross-Border Healthcare Directive, a new EU Directive outlining patients' rights to access treatment abroad, was implemented in 2011. The European Commission recently issued a call for the creation of a network of reference centres in paediatric haematology/oncology to which SIOPE responded. Based upon ongoing EU funded projects such as ENCCA [10], PanCareSurFup [14] and INTREALL [15], SIOPE along with 42 institutions and organisations proposed to build a reference network named ExPO-r-Net for expert centres in Paediatric Oncology which will integrate the SIOPE's European Standard of Care initiative. The proposal has been selected and funded for 3 years (Table 4) and will be launched in 2014.
TABLE IV.
The Goals and Output of the ExPO-r-Net Project (European Expert Paediatric Oncology Reference Network for Diagnostics and Treatment)
| Goal | Output/field | |
|---|---|---|
| 1 | Identify the medical needs of rare children and adolescents with cancer with experts of the European Council of Clinical Research and address the challenges in terms of costs, resources, psychological burden and ethical aspects | Paediatric needs |
| 2 | Build a Paediatric Oncology European Research Network (ERN)—a roadmap to identified and certified reference expert centres | Network |
| 3 | Establish a Paediatric Oncology tumour board ERN working to common standards and using IT tools based on E-Health concepts for sharing and providing expertise and advice | Tumour boards |
| 4 | Define the criteria for a common process for identification and designation of paediatric oncology expert centres in Europe | Criteria for designation |
| 5 | Address the cross-border dimension of long-term follow-up of childhood cancer survivors in Europe: the survivorship passport as an instrument for crucial treatment and follow-up data | Long-term follow-up |
| 6 | Integrate very rare tumours and soft tissue sarcomas into the European reference network | Very rare tumours |
Paediatric oncology nurses are essential members of the multidisciplinary teams taking care of children and adolescents. They provide high quality, safe and effective nursing care which is paramount to warrant access to standard treatments and the best quality of life possible. There is a need to facilitate the implementation of nursing research projects addressing, for example and not limited to, symptoms management and quality of life of patients and their families during treatment [16]. There is a need to increase the role of nurses in clinical research which is integrated into care and SIOPE will develop further the collaboration with European paediatric oncology nurses.
Most paediatric malignancies develop rather rapidly as compared to adult malignancies. Early diagnosis through large national programmes is thus difficult to achieve with the exception of the small minority of children and adolescents recognized to be at increased genetic risk of developing a cancer. This is in contrast to adult cancer screening programmes in breast, cervical and colo-rectal cancers. For some patients, there is a long delay between the first symptoms and diagnosis of cancer that delays initiation of treatment and may jeopardise patient outcome. This may be disease related [17]. Headsmart is a pilot project currently running in the UK to reduce delays in diagnosis of brain tumours [18]. This is a national public health campaign to raise awareness of the early features of brain tumours among the public, schools and primary care practitioners and the need for timely imaging.
IMPROVING LONG-TERM FOLLOW-UP
With a 80% cure rate, the number of survivors, presently estimated to be 300,000–500,000 in Europe, is likely to continue to increase and improving their quality-of-life is a major goal [19]. To address this, the PanCare network was created in 2008 [14]. PanCare is a pan-European multidisciplinary network of health professionals, survivors of a paediatric malignancy and their families, collaborating to reduce the frequency, severity and impact of late side-effects of treatments, with the aim to ensure that every survivor of childhood cancer receives an optimal long-term care. PanCare increases awareness and facilitates research about childhood cancer survivors, as well as working towards promoting health organisations to address the issues of long-term follow-up. From within PanCare, 16 institutions from 11 European countries formed the PanCareSurFup consortium which was selected and funded within the Seventh Framework Programme, at the same time as ENCCA. PanCareSurFup carries out research on late effects such as the effects of radiation therapy, cardiac toxicity and secondary malignancies. Guidelines for follow-up, models of care and transition are being established as well as the training needs of health professionals. A Survivorship passport is being developed in ENCCA in collaboration with PanCareSurFup aiming to provide each cured child and adolescent with information on treatment received and the relevant follow-up, including precautionary measures to improve their quality-of-life despite the potentially toxic treatment they received. The hope is to empower survivors to take the responsibility for their own follow-up ensuring that they are well-informed on what to be aware of, how and when to access care and follow-up and who to turn to when and if they need and desire. In 2014, PanCareLIFE, another PanCare-based 5-year EU-project, will commence. PanCareLIFE will focus on fertility and ototoxicity issues as well as quality-of life after childhood cancer.
TRAINING MULTIDISCIPLINARY TEAMS
The quality of standard care delivered to children and adolescents with cancer relies on well-trained multidisciplinary teams. There are several European training and education programmes such as those delivered by the European School of Oncology, the Amsterdam School of Paediatric Oncology, the Innovative Therapies for Children with Cancer and the Diplome Inter-Universitaire d'Oncologie Pédiatrique. In addition, SIOPE supports the annual Flims Workshop on methods in clinical cancer research. This highly competitive educational programme introduces junior clinical oncologists in any oncology subspecialty to the principles of good clinical trial design.
SIOPE prepared a comprehensive new syllabus in paediatric haematology/oncology in collaboration with the European Academia of Paediatrics [20]. The goal is to ensure a standard training programme throughout Europe, allowing specialists to be qualified in paediatric haematology/oncology to exercise their skills in a specialised tertiary care unit. The Syllabus sets out the minimum requirements for a Europe-wide 2-year training programme. It is designed in a modular fashion, covering haematological malignancies and solid tumours, diagnostic, therapeutic approaches, research methodologies and ethical issues as well as expertise in practical procedures.
SIOPE has also developed in partnership with ECCO, three educational videos providing accredited online educational resources for young oncologists who need practice-oriented training. The videos explain the standard procedures in paediatric haematology/oncology such as lumbar puncture, bone marrow aspiration and bone marrow biopsy.
PARTNERING WITH PARENT, PATIENT AND SURVIVOR ORGANISATIONS
Building a partnership with parents, patients and survivors organisations is essential to achieve the goals in terms of research, equal access to standard care and expertise, advocacy when new policies are developed and strategic decisions regarding priorities to be undertaken at the national and European levels. SIOPE has been working with ICCCPO for several years and ICCCPO is involved as a partner in several European projects and initiatives.
ICCCPO is a worldwide childhood cancer organization representing families of children with cancer and childhood cancer survivor groups [21]. In addition, paediatric cancer survivors are starting to create organisations, such as “Les Aguerris” [22] in France, SurvivorNet [23] in the UK and UngCancer [24] in Sweden and to build European networks. In 2011, ICCCPO European members and survivors created the Parent and Patient Advocacy Committee (PPAC) within ENCCA. Since that time they have actively engaged in facilitating the networking between parent and survivor groups and organisations, building a strong network of survivor groups and disseminating health policy related issues within Europe. The priorities are (i) closing the gap' in research, treatment and care between Western and Eastern Europe, (ii) the training of parent and patient representatives in clinical research to make them powerful advocates and partners in this field, (iii) survivors' issues and long-term follow-up (including the survivorship passport), (iv) contribution to the definition and implementation of strategic objectives within Europe for the next 15 years.
ADVOCATING NEW EUROPEAN ONCOPOLICY INITIATIVES
The EU Clinical Trials Directive (CTD) has significantly impaired the capacity to run academic clinical trials due to the substantially increased administrative burden and costs. As a Directive, the CTD has been implemented differently by Member States, generating substantial differences of interpretation, process and priority across Europe, severely complicating the tasks for academic sponsors running multinational clinical trials. Such trials are essential for paediatric haematology/oncology, given the rarity of childhood cancers. Consequently the number of clinical trials recruiting patients to test new treatments has decreased significantly in Europe [25]. Instead of revising the CTD, the European Commission proposed a new legislation in the form of a Regulation which will apply on the same day, in each EU Member State without any transposition [26]. SIOPE applauded this decision and worked closely with the European Commission, the European Parliament and the European Council to try and influence the content of the Regulation so that it meets the needs of those running trials for young people with rare cancers [27]. The critical points which have been selected and strongly endorsed by SIOPE to ensure that the new Regulation will be an improvement include: (i) the need for simplified administrative process and reporting for clinical trials with limited additional risk compared to normal clinical practice, categorizing the risk linked to research rather than the current “one size fits all” approach, (ii) the need for national indemnity schemes to avoid the current situation where disproportionately high insurance fees need to be raised by academia to cover investigator driven academic clinical trials.
The European Paediatric Medicines Regulation that was launched in 2007 to provide better medicines for children is a highly relevant legislation for paediatric haematology/oncology [28]. This Regulation is based on rewards, incentives and obligations for pharmaceutical companies to develop drugs for young people. There were high expectations that this legislation would bring hope to children suffering with cancer with the possibility that anticancer drugs for adults could also be developed for children [29]. After 5 years, there has been a significant change in the landscape of paediatric haematology/oncology drug development in Europe and several new anticancer drugs are under investigation. However, there is still an insufficient access to new drugs. SIOPE along with ITCC and parent organisations have voiced their concerns on limitations of the implementation of the Paediatric Regulation in oncology during the Public Consultation process on the 5-year interim report, prepared by the European Medicines Agency in 2012. We supported proposals by the EMA [30] and asked for a revocation of the class waiver list, the approval of PIPs on the basis of the drug mechanism of action rather than the adult indication along with a better prioritisation of drug development, in order to make the Regulation successful in addressing the urgent need for new safe and effective drugs in paediatric haematology/oncology [31]. We will continue to advocate for better incentives and regulation to cover the specific needs of children and adolescents with cancer. This will become a very important activity for SIOPE in the coming years.
In 2014, the EU launched Horizon 2020, the next EU framework program for research and innovation, with three objectives: excellence in science, competitive industries and tackling societal challenges. SIOPE will make all efforts to assure that needs of young people with cancer are adequately addressed.
BEING PART OF THE GLOBAL AGENDA AGAINST CHILDHOOD CANCERS
Several European-centred study groups are international in their scope and bring together global investigators to participate in a common clinical trials portfolio. The IBFM-study group is international and patients from countries outside Europe, such as Argentina or Japan, participate to the IBFM leukaemia trials and translational research projects [32]. Today, more than 100 institutions from Europe, Asia, Central and South America, Australia and New Zealand collaborate in SIOPEL, the SIOPE-Liver study group. Early drug trial groups such as ITCC in Europe, POETIC and TACLE in North America, the Canadian C17 network and the Australia Children's Cancer Trials group are working together to speed up the development of new anticancer agents [33]. SIOPEN built with the Children's Oncology Group and neuroblatoma groups from Japan, China and Australia a large clinical and biological database of more than 9,000 neuroblastoma patients in order to define and validate new staging system, new biological prognostic biomarkers through the International Neuroblastoma Research Group [34]. Groups such as the SIOP-Renal Tumours Study Group support clinical trials groups in other continents to run studies using the same standard treatment backbones, adapted to local circumstances [35].
CONCLUSION
Cancer in young people remains an important global public health issue. Only cooperation between the European paediatric haematology/oncology community of health professionals, researchers, parents, patients and survivors together with all stakeholders (national and European political bodies, regulatory agencies, pharmaceutical companies, fund raising charities) and including international collaborations will overcome the challenges to increase both cure and quality of cure of children and adolescents with cancer and to warrant long term sustainability.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5-a population-based study. Lancet Oncol. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard-Jones K, Pieters R, Reaman GH, et al. Sustaining innovation and improvement in the treatment of childhood cancer: Lessons from high-income countries. Lancet Oncol. 2013;14:e95–e103. doi: 10.1016/S1470-2045(13)70010-X. [DOI] [PubMed] [Google Scholar]
- 4.Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, et al. Cancer in children and adolescents in Europe: Developments over 20 years and future challenges. Eur J Cancer. 2006;42:2183–2190. doi: 10.1016/j.ejca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Calaminus G, Birch JR, Hollis R, et al. The role of SIOP as a platform for communication in the global response to childhood cancer. Pediatr Blood Cancer. 2013;60:2080–2086. doi: 10.1002/pbc.24728. [DOI] [PubMed] [Google Scholar]
- 6.The European Cancer Organisation. www.ecco-org.eu (accessed on 1st November 2013)
- 7.Schilsky RL. Personalized medicine in oncology: The future is now. Nat Rev Drug Discov. 2010;9:363–366. doi: 10.1038/nrd3181. [DOI] [PubMed] [Google Scholar]
- 8.Marris S, Morgan S, Stark D. Listening to Patients”: What is the value of age-appropriate care to teenagers and young adults with cancer. Eur J Cancer Care (Engl) 2011;20:145–151. doi: 10.1111/j.1365-2354.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan R, Kowalczyk JR, Agarwal B, et al. New policies to address the global burden of childhood cancers. Lancet Oncol. 2013;14:e125–e135. doi: 10.1016/S1470-2045(13)70007-X. [DOI] [PubMed] [Google Scholar]
- 10.The European Network for Cancer research in Children and Adolescents. www.encca.eu (accessed on 1st November 2013)
- 11.The European Partnership for Actions Against Cancer. www.epaac.eu (accessed 1st November 2013)
- 12.The Eurocanplatform. www.eurocanplatform.eu (accessed 1st November 2013)
- 13.Kowalczyk JR, Samardakiewicz M, Fitzgerald E, et al. Towards reducing inequalities: European Standards of Care for Children with Cancer. Eur J Cancer. 2014;50:481–485. doi: 10.1016/j.ejca.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.PanCare Childhood and Adolescent Cancer Survivor Care and Follow-Up Studies (PanCaerSurFup) www.pancaresurfup.eu (accessed 1st November 2013)
- 15.The International Study for treatment of Childhood relapsed acute lymphoblastic leukaemias. http://www.intreall-fp7.eu (accessed 1st November 2013)
- 16.Maru M, Gibson F, Hinds PS. Pediatric oncology nursing research goes global. Cancer Nurs. 2013;36:339. doi: 10.1097/NCC.0b013e3182a34688. doi: 10.1097/NCC.0b013e3182a34688. [DOI] [PubMed] [Google Scholar]
- 17.Brasme JF, Morfouace M, Grill J, et al. Delays in diagnosis of paediatric cancers: A systematic review and comparison with expert testimony in lawsuits. Lancet Oncol. 2012;13:e445–e459. doi: 10.1016/S1470-2045(12)70361-3. [DOI] [PubMed] [Google Scholar]
- 18.Headsmart-be brain tumour aware. www.headsamart.org.uk (accessed 1st November 2013)
- 19.Rowland JH, Kent EE, Forsythe LP, et al. Cancer survivorship research in Europe and the United States: Where have we been, where are we going, and what can we learn from each other. Cancer. 2013;119:2094–2108. doi: 10.1002/cncr.28060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The SIOPE syllabus—European training programme in paediatric haematology and oncology. http://www.siope.eu/wp-content/uploads/2013/11/European-training-Paediatrics_New.pdf (accessed 1st November 2013) [DOI] [PubMed]
- 21.The International Confederation of Childhood Cancer Parents Organisation. http://www.icccpo.org.
- 22.Les Aguerris. www.lesaguerris.wordpress.com (accessed 1st November 2013)
- 23.SurvivorNet. http://SurvivorNet.org (accessed 1st November 2013)
- 24.UngCancer. www.ungcancer.se (accessed 1st November 2013)
- 25.Hartmann M. Impact assessment of the European Clinical Trials Directive: A longitudinal, prospective, observational study analyzing patterns and trends in clinical drug trial applications submitted since 2001 to regulatory agencies in six EU countries. Trials. 2012;13:53. doi: 10.1186/1745-6215-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willmott G. Regulating clinical trials in Europe. Lancet Oncol. 2013;14:453–454. doi: 10.1016/S1470-2045(13)70071-8. [DOI] [PubMed] [Google Scholar]
- 27.Kearns P. The need for proportionate regulation of clinical trials. Lancet Oncol. 2013;14:454–455. doi: 10.1016/S1470-2045(13)70077-9. [DOI] [PubMed] [Google Scholar]
- 28.Olski TM, Lampus SF, Gherarducci G, et al. Three years of paediatric regulation in the European Union. Eur J Clin Pharmacol. 2011;67:245–252. doi: 10.1007/s00228-011-0997-4. [DOI] [PubMed] [Google Scholar]
- 29.Vassal G, Geoerger B, Morland B. Is the European pediatric medicine regulation working for children and adolescents with cancer. Clin Cancer Res. 2013;19:1315–1325. doi: 10.1158/1078-0432.CCR-12-2551. [DOI] [PubMed] [Google Scholar]
- 30.Saint-Raymond A, Herold R. Medicines for pediatric oncology: Can we overcome the failure to deliver. Expert Rev Clin Pharmacol. 2012;5:493–495. doi: 10.1586/ecp.12.51. [DOI] [PubMed] [Google Scholar]
- 31.Vassal G, Blanc P, Pearson A. Need for change in implementation of paediatric regulation. Lancet Oncol. 2013;14:1156–1157. doi: 10.1016/S1470-2045(13)70467-4. [DOI] [PubMed] [Google Scholar]
- 32.The International BFM study group. www.bfm-international.org (accessed 1st November 2013)
- 33.Vassal G, Zwaan CM, Ashley D, et al. New drugs for children and adolescents with cancer: The need for novel development pathways. Lancet Oncol. 2013;14:e117–e124. doi: 10.1016/S1470-2045(13)70013-5. [DOI] [PubMed] [Google Scholar]
- 34.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malogolowkin M, Spreafico F, Dome JS, et al. Incidence and outcomes of patients with late recurrence of Wilms' tumor. Pediatr Blood Cancer. 2013;60:1612–1615. doi: 10.1002/pbc.24604. [DOI] [PubMed] [Google Scholar]


