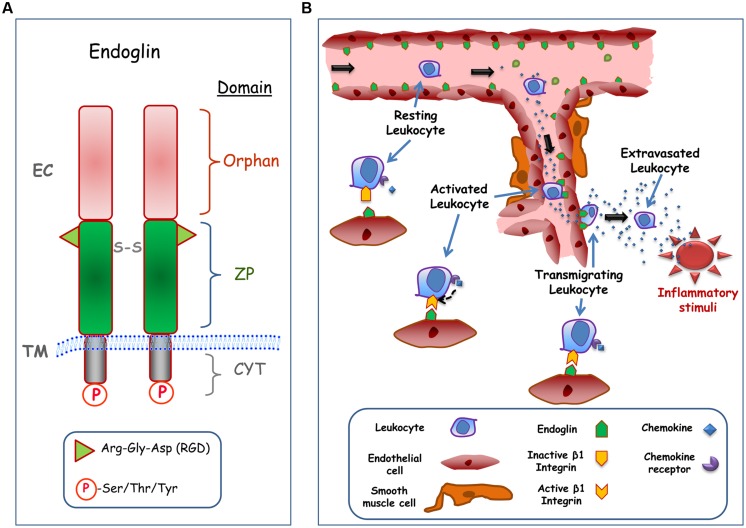
FIGURE 1.
Structure and cell adhesion function of endoglin. (A) Structural representation of endoglin. Endoglin is a type I membrane protein with a large extracellular (EC) domain that contains a zona pellucida (ZP) domain in the juxtamembrane region and an NH2-terminal orphan domain. The ZP domain encodes an Arg-Gly-Asp (RGD) tripeptide that is involved in integrin binding, whereas de orphan domain is involved in binding to members of the TGF-β superfamily. Endoglin forms dimers and the corresponding monomers are disulphide linked (S-S). The cytoplasmic (CYT) domain can be phosphorylated (P) at Ser/Thr/Tyr residues. The transmembrane (TM), and EC domains of the protein are indicated. The scheme is not to scale. (B) Role of endothelial endoglin in leukocyte adhesion and transmigration. A schematic diagram shows a hypothetical model for leukocyte transmigration through the vessel endothelium. In an inflammatory focus, different soluble factors are released, including the chemokine CXCL12, leading to activation and endoglin-dependent extravasation of leukocytes. The transmigration process of the leukocyte involves the binding of CXCL12 to its receptor CXCR4, which in turn activates β1 integrins. Once activated, β1 integrin binds to the RGD motif of endoglin present on the endothelial cell surface, allowing the extravasation and migration of leukocytes to the inflammatory site.