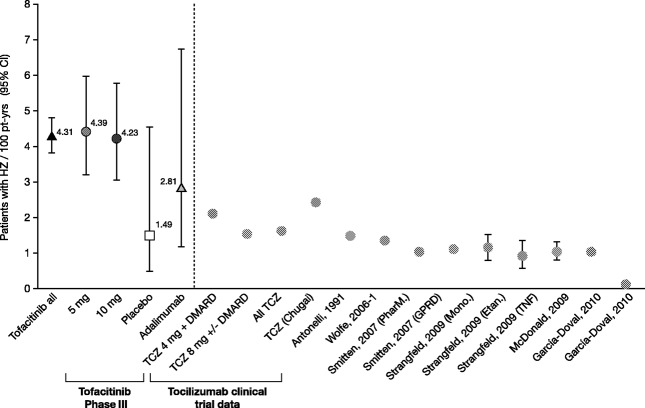
Figure 1.
Crude incidence rates of herpes zoster (HZ) in the Tofacitinib Rheumatoid Arthritis (RA) Development Program (left of broken line) and in published studies of patients with RA treated with nonbiologic and biologic disease-modifying agents (right of broken line) (see refs.5 and24–30). HZ incidence rates (with 95% confidence intervals [95% CIs]) are expressed per 100 patient-years of exposure. The Tofacitinib all group comprises data from tofacitinib-treated patients in the phase II, phase III, and long-term extension studies of the development program. The Tofacitinib phase III group comprises data from the phase III studies in the 5 mg tofacitinib twice daily, 10 mg tofacitinib twice daily, placebo, and adalimumab exposure groups. The Tocilizumab (TCZ) clinical trial data group comprises data from patients treated with 4 mg TCZ plus disease-modifying antirheumatic drugs (DMARDs), those treated with 8 mg TCZ with or without DMARDs, all TCZ-treated patients, and TCZ-treated patients reported in the US Food and Drug Administration documents submitted by Chugai (29,30). PharM. = PharMetrics database; GPRD = General Practice Research Database; Mono. = monoclonal anti–tumor necrosis factor α (anti-TNFα) antibodies (adalimumab and infliximab); Etan. = etanercept.