fig. 2.
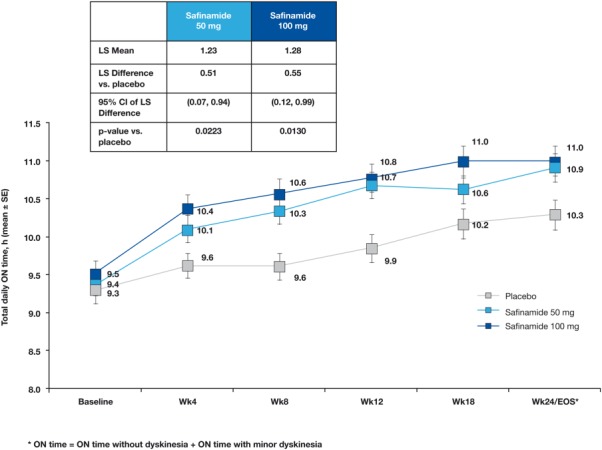
Primary endpoint. Mean change ± SE in on time with no or minor dyskinesia during the study (patient diary data). Using ANCOVA analysis (MMRM), all time points after baseline were statistically significant when compared with placebo, with the exception of safinamide 50 mg/day at week 18 (P = 0.0974). *P < 0.05 versus placebo. ANCOVA, analysis of covariance; MMRM, mixed model repeated measures; LS, least squares; CI, confidence interval; EOS, end of study; SE, standard error.
