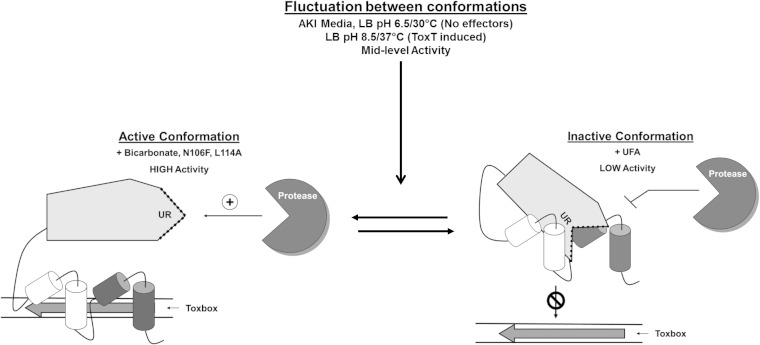
FIG 14.
Model for effector control of ToxT activity. (Left) High-level transcriptional activation by ToxT with the addition of positive effector bicarbonate. ToxT undergoes conformational change with the addition of bicarbonate that allows increased DNA binding affinity to toxbox and leaves the unstructured region (UR) accessible by protease. The active conformation resembles those of ToxT N106F and L114A mutants. (Right) Low-level transcriptional activation by ToxT with the addition of unsaturated fatty acids (UFA). Upon binding UFA, ToxT takes on an inactive conformation that results in low toxbox binding affinity and decreased proteolysis due to unexposed unstructured region. (Center) Midlevel transcriptional activation by ToxT in the absence of effector molecules. ToxT fluctuates between inactive and active conformations, leading to potential ToxT proteolysis. The culture conditions used were AKI without added bicarbonate, virulence-inducing growth conditions (LB, pH 6.5, 30°C, shaking) without negative effectors, or virulence-repressing growth conditions (LB, pH 8.5, 37°C, shaking) for ToxT induction in trans.