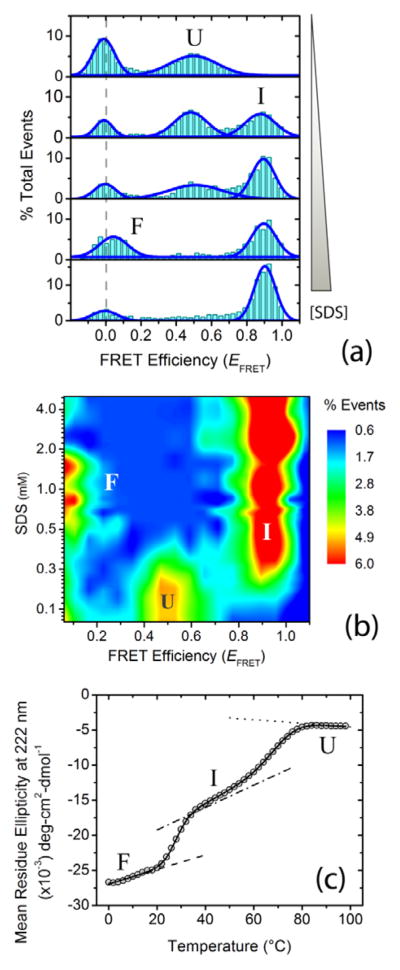
Figure 1.
Ligand-induced multistate folding of the intrinsically disordered protein α-synuclein. Single-molecule and ensemble experiments were performed in αβγ buffer (i.e., 0.2 M sodium chloride, 10 mM sodium acetate, 10 mM monosodium phosphate, 10 mM glycine) at pH 10.5 ± 0.1. (a) Representative single-molecule Förster resonance energy transfer (smFRET) histograms as a function of increasing concentration (0, 0.2, 0.3, 0.6 and 5.0 mM) of the ligand and lipid mimic sodium dodecyl sulfate (SDS). The dashed gray line shows the expected zero peak center position. Peaks for U, I and F states are indicated. (b) [Ligand]-FRET efficiency (EFRET) contour map, color-coded on the basis of fractional population. (c) Thermal unfolding of α-synuclein folded using 1mM SDS, monitored using far-UV CD spectroscopy. The solid curve represents the nonlinear least-squares (NLS) fit of the data to a three-state model;[11] the dotted, dash-dotted and dashed lines are the baselines for U, I and F states.