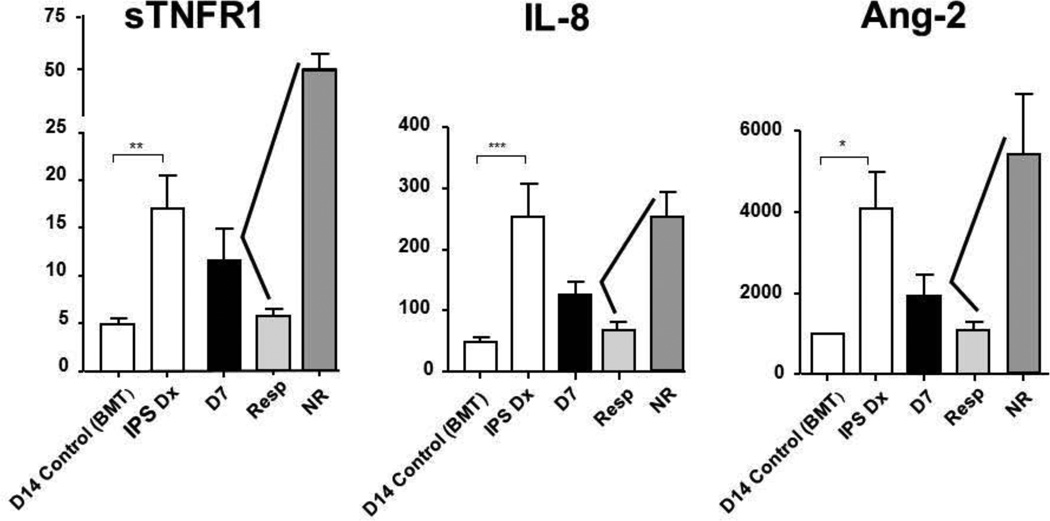
Figure 3.
Plasma biomarker levels of IPS at the time of diagnosis and on day 7 of study therapy, in responders and non-responders.
Plasma protein levels were analyzed from patient samples collected at the time of IPS diagnosis (IPS Dx), and on day 7 (D7) of study. Day 7 levels are then subdivided by those patients that responded (Resp) or did not-respond (NR) to study therapy. Control samples were obtained on day 14 post-transplant in allogeneic transplant recipients without complications [D14 Control (BMT)]. Data are expressed as mean ± SEM. Significant differences between groups are shown as: * p<0.05, ** p< 0.01, *** p<0.001