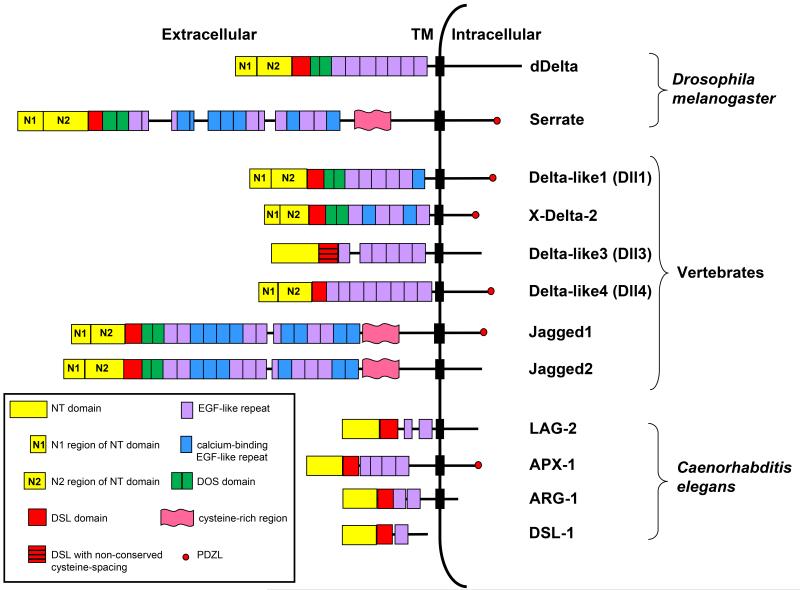
Fig. 2. Structural domains of canonical ligands.
The extracellular domains of canonical ligands are characterized by the presence of an N-terminal (NT) domain followed by a Delta/Serrate/LAG-2 (DSL) domain and multiple tandemly arranged Epidermal Growth Factor (EGF)-like repeats (see text for details). The DSL domain together with the flanking NT domain and the first two EGF repeats containing the Delta and OSM-11-like proteins (DOS) motif are required for canonical ligands to bind Notch. The NT domain of vertebrate and Drosophila ligands is subdivided into a region containing six conserved cysteine residues, N1 and a cysteine- free region, N2. Serrate/Jagged ligands contain an additional cysteine-rich region not present in Delta-like ligands. The intracellular domains of some canonical ligands contain a carboxy-terminal PSD-95/Dlg/ZO-1-ligand (PDZL) motif that plays a role independent of Notch signaling. C. elegans DSL ligands lack a DOS motif but have been proposed to cooperate with DOS-only containing ligands (not depicted) to activate Notch signaling. Dll3 is the most structurally divergent vertebrate DSL ligand and lacks structural features required by other DSL ligands to bind and activate Notch.