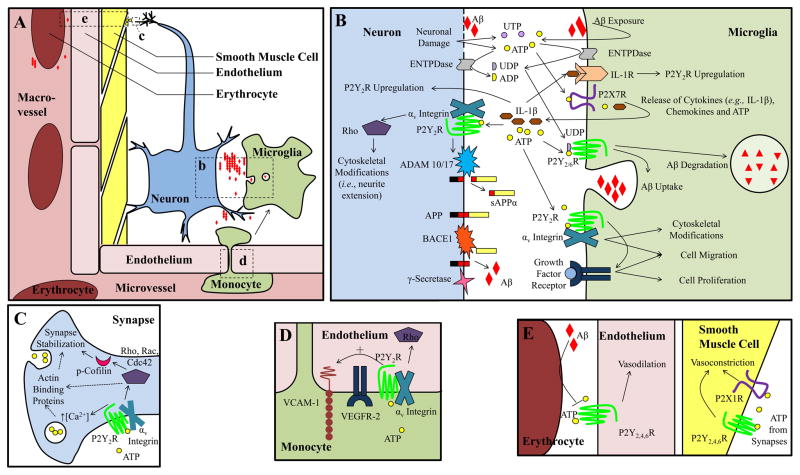
Figure 2. P2Y receptor function in AD.
A. Overview of cell types involved in neuroinflammation and the neurovascular unit. Areas b–e in panel A are magnified in panels B–E. Although intimately involved, neuron-associated astrocytes and oligodendrocytes and microvessel-associated pericytes are not shown for simplicity. Aβ alters cellular release of nucleotides, including increased ATP release from microglia and decreased ATP release from hypoxic erythrocytes. In perivascular neurons, ATP is released from synaptic vesicles and causes vasoconstriction by activating P2X1 and P2Y2,4,6 receptors on vascular smooth muscle cells. P2Y1,2,4,6 receptors in vascular endothelial cells promote vasodilation by responding to ATP in the blood stream. The endothelial P2Y2R facilitates monocyte adhesion to the vascular wall, through VEGFR-2-dependent upregulation of vascular cell adhesion molecule-1 (VCAM-1) as well as monocyte extravasation. Activation of neuronal P2Y1,2 receptors promote neurite extension and stabilization, whereas P2Y13R inhibits neurite extension. Neuronal P2Y2R facilitates non-amyloidogenic processing of APP through ADAM10/17-dependent production of soluble APPα (sAPPα). P2Y1,12,13 receptors enhance glycine transport in the synaptic cleft and P2Y11R activation in glutamatergic neurons delays apoptosis. P2YRs increase dopamine and glutamate release, but can also inhibit neurotransmitter release in the cerebral cortex and hippocampus. In microglia, P2Y1,2,6,12,13 receptor activation increases microglial cell migration and P2Y2,6 receptors promote Aβ uptake and degradation. In astrocytes, P2Y1,6 receptors stimulate the production of proinflammatory cytokines and chemokines, P2Y2,4 receptors increase the production and secretion of APP and P2Y14R increases expression of MMP9, which degrades Aβ.