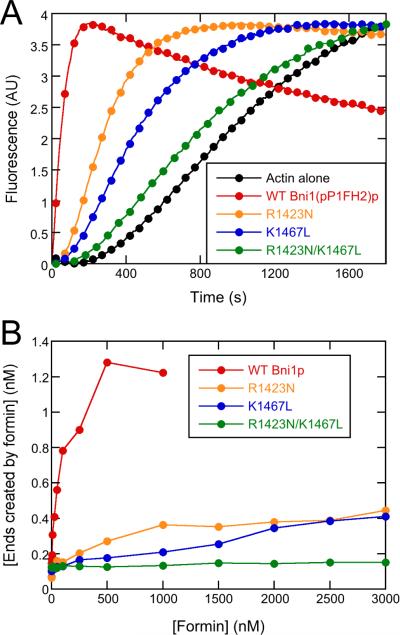
Figure 8. Substitutions R1423N and K1467L of Bni1(pP1FH2)p strongly impair actin filament nucleation.
Conditions: 10 mM imidazole (pH 7.0), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.17 mM ATP, 0.5 mM DTT, 0.03 mM CaCl2, 1.7 mM Tris (pH 8.0), 1 part per 105 (w/v) Antifoam 204. (A) Representative time courses of spontaneous polymerization of 4 μM actin (20% pyrene-labeled) alone or in presence of 1 μM wild-type or mutant Bni1(pP1FH2)p constructs. For clarity, every 5th data point is shown. (B) Dependence of the concentration of formin-nucleated barbed ends on the concentration of Bni1(pP1FH2)p constructs. Barbed end concentrations were calculated from the elongation rates in bulk samples when half of the total actin was polymerized and the elongation rates measured in the TIRF microscopy experiments. See also Figures S5 and S6 for additional information about the experimental characterization of the actin/formin system.