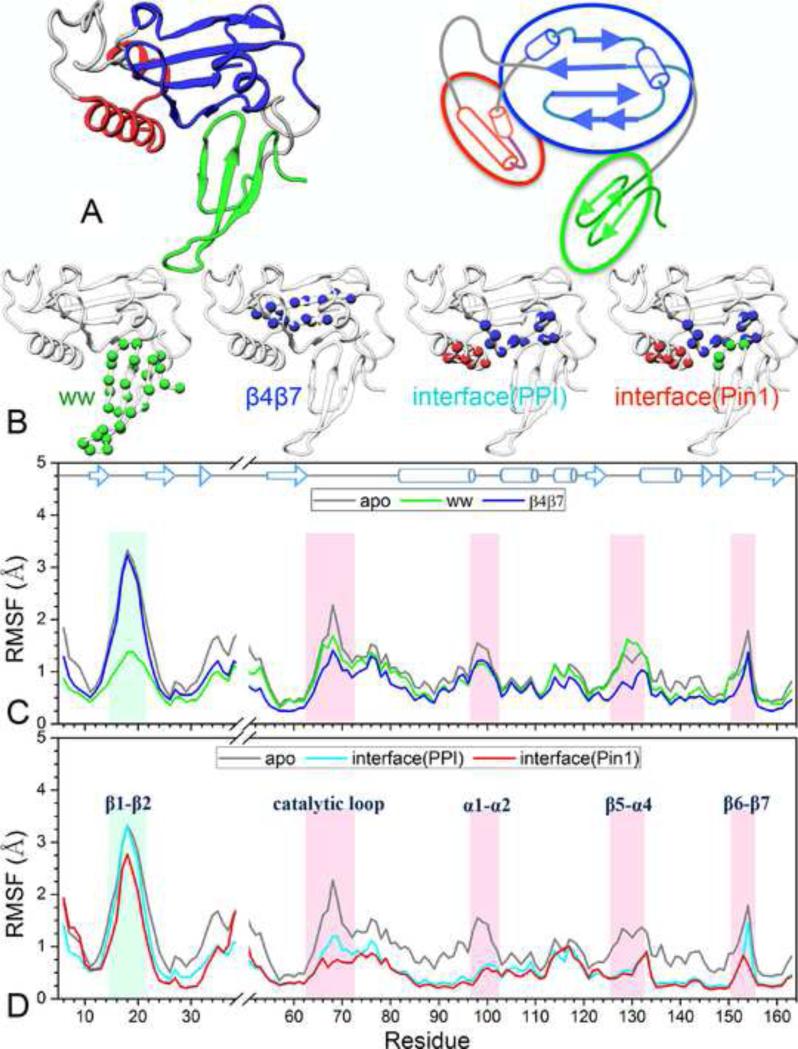
Figure 5. Design and results of the restrained simulations.
(A) Pin1 as a tripartite molecule, with its three modules, the WW domain, the PPIase core, and the α1-α2 appendage, shown in green, blue, and red, respectively. Left: 3-dimensioanl structure; right: schematic representation.
(B) Four sets of intra-module (shown as Cα spheres in single color) and inter-module (shown as Cα spheres in mixed color) restraints. The restrained residues are: WW, residues 6-34; β4β7, residues 55-62 and 156-161; interface(PPI), residues 86, 87, 90, 91, 93, 94, 97, 136-138, 140-142, and 145-151; interface(Pin1), all interface(PPI) residues and residues 28-31.
(C,D) The Cα RMSFs of the four restrained simulations, compared to those of the unrestrained apo Pin1 simulation.