Fig. 3. The interactions of Sox and bHLH genes in neurosensory differentiation of a mouse.
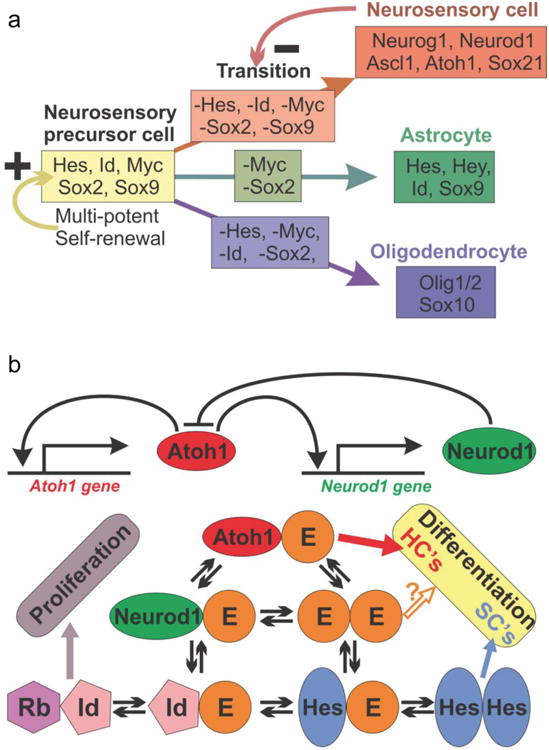
Experimental data in mice have shown a complex interaction of Sox genes and bHLH genes in the progression of neurosensory cell fate commitment and differentiation. a) Sox2 and Sox9 are essential genes in neurosensory precursor cells that ensure self-renewal of precursors but also commitment to the neurosensory lineage. This appears to be in interaction with several bHLH genes that are later found in astrocytes (Hes, Hey, Id). Virtually all of these neurosensory precursor genes are turned off in the neurosensory lineage (arrow with – on transition) and mostly bHLH genes are activated, including Sox21 that antagonizes Sox2. Oligodendrocyte precursors also shut off neurosensory precursor genes but are characterized by a different set of bHLH genes (Olig1/2) and Sox10 and Sox2 for terminal differentiation. bHLH transcription factors can form complex interactions in a given cell that can undergo periodic changes (oscillates) and their signal can undergo context dependent variation between gene expression and suppression. Data in mice and flies suggest that all proneural transcription factors compete for the E-proteins (Tcf3,4,12) to form heterodimers for proper binding. Thus, the level of all proneuronal bHLH TFs (here Atoh1 and Neurod1) and available E-proteins as well as their binding preference will determine how much signaling of heterodimers will occur. Importantly, E-proteins can also interact with Hes/Hey factors and the inhibitors of DNA binding (Ids), limiting availability of E-proteins for proneuronal protein heterodimerization, proportionally to the affinity and concentration of all these interactive partners. In essence, the binding properties and frequency of the binding partners will determine whether a cell is differentiating as a neuron/hair cell, a supporting/glial cell, or is continuing proliferation as a prosensory precursor. HC, hair cell and SC, supporting cell. Modified after (Forrest, et al., 2014, Imayoshi and Kageyama, 2014, Pan, et al., 2012, Reiprich and Wegner, 2014).
