Figure 2.
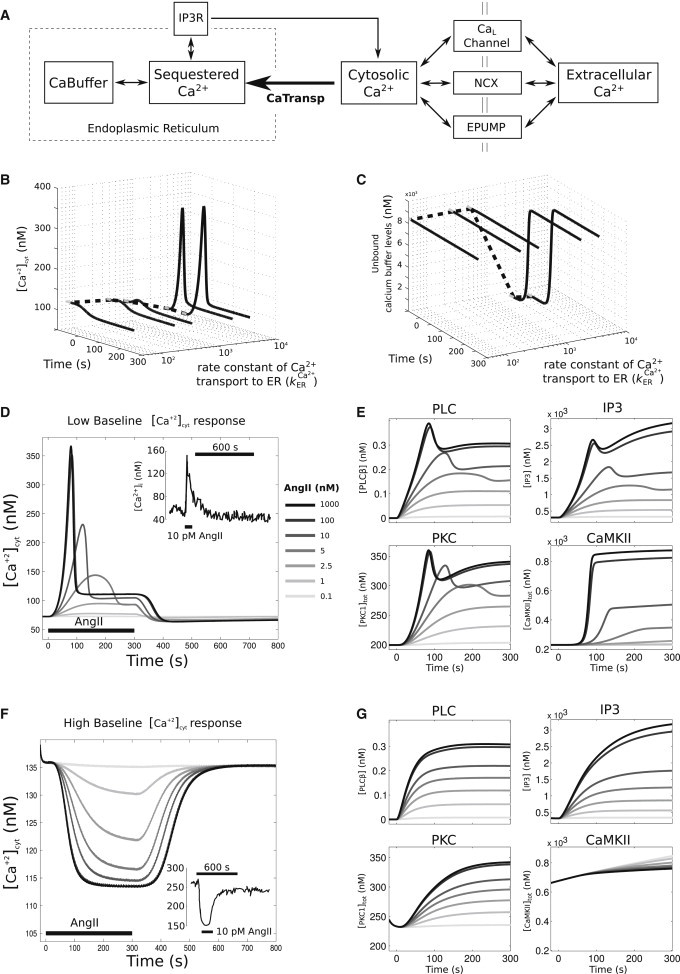
Nonlinear baseline Ca2+-dependent response to AngII stimulus, in which low cytosolic Ca2+ baseline levels resulted in an increase in cytosolic Ca2+ and high cytosolic Ca2+ levels resulted in a decrease in cytosolic Ca2+ levels. (A) Schematic of Ca2+ regulatory dynamics. (B) Dynamic traces of cytosolic Ca2+ as a function of change in the rate constant of a Ca2+ transport into the ER . (Solid lines) Time period where 100 nM AngII was applied; (shaded lines) steady-state levels preceding AngII application. Decreasing the from 3600 to 36 μm−2 s−1 increases the Ca2+ baseline levels from ∼72 to 136 nM (dotted line). (C) Dynamic traces of unbound calcium buffer levels as a function of change in . For plot details, see (B). Decrease in drains out all stored Ca2+ in the buffer before AngII application (total capacity of the saturated buffer = 9091 nM). (D) AngII dose-dependence of cytosolic Ca2+ response for low cytosolic Ca2+ baseline condition. (Inset, top right) Intracellular calcium tracing from primary cultures of neonatal rat sympathetic neurons when exposed to 3 min AngII pulses (10 pM) for low baseline condition (27). (E) Upstream responses of PLCβ and IP3, and downstream kinase responses of PKC and CaMKII for the low Ca2+ baseline condition. (F) AngII dose-dependence of cytosolic Ca2+ response for high cytosolic Ca2+ baseline condition. (Inset, bottom-right panel) Intracellular calcium tracings taken from cells (see D for details) with a higher baseline (>200 nM). (G) High Ca2+ baseline condition upstream responses of PLCβ and IP3, and downstream kinase responses of PKC and CaMKII.
