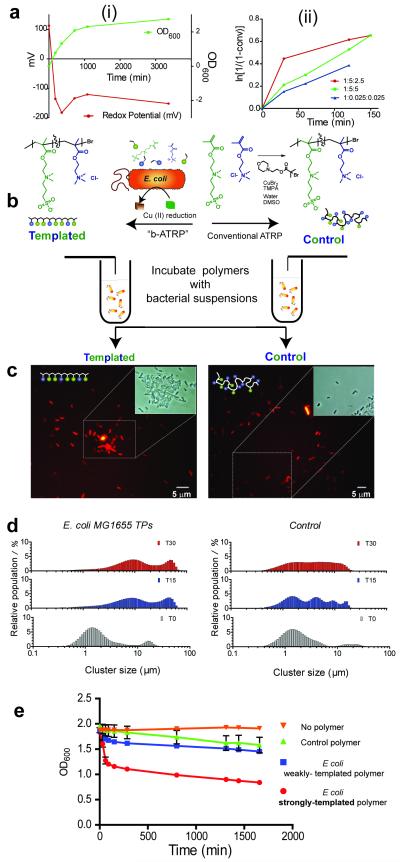
Figure 2. Generation of a reductive environment during bacteria-instructed synthesis and evaluation of the cell-binding properties of the resultant polymers.
Polymers grown in the presence of bacteria (‘templated’) exhibit different properties compared to those grown in the same conditions but without the cells (‘control’). In (A) are shown (i) the changes in redox potential of suspensions (red line) as E. coli cells proliferate and enter stationary phase (optical density at 600 nm, green line), and in (ii) the kinetics of polymer growth in E coli suspensions at different ratios of polymerisation initiator: copper (II): ligand. In (B) the templating process is shown schematically, while in (C), fluorescence microscopy shows clusters of mCherry-labelled bacteria in presence of templated polymer, indicating a high affinity of the polymer for the cell type by which it was templated. This is in contrast to the isolated cells observed after incubation with control polymer after 30 min (insets show phase contrast images from the selected sections to show depth of clustering). In (D) bacterial aggregation is quantified via Coulter counter analysis of polymer-bacteria clusters: in D(i) ~ 50 μm clusters are apparent within 15 min for the templated species in contrast to the lack of cluster formation (D(ii) for the control polymers. In (E) optical density measurements confirm rapid binding and sequestration of E. coli by polymers grown in the presence of E. coli and recovered from the cells by a high salt wash step (‘strongly-templated’ polymers).