Figure 2.
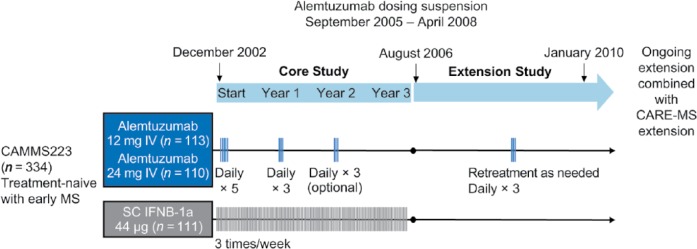
CAMMS223 phase II study design. Alemtuzumab was infused intravenously (IV) on 5 consecutive days at baseline and on 3 consecutive days at year 1. The third treatment course at year 2 was given at the discretion of the investigator if CD4+ T-cell counts were ⩾100 × 106 cells/l. In the extension study, patients originally randomized to SC IFNB-1a were not eligible for alemtuzumab treatment, but could take other disease-modifying therapies. Patients originally randomized to alemtuzumab could receive alemtuzumab retreatment at any point during the extension after the dosing suspension was lifted and ⩾12 months after the previous treatment course. Only the 12-mg dose was used for retreatment.
CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; IFNB, interferon beta; MS, multiple sclerosis; SC, subcutaneous.
