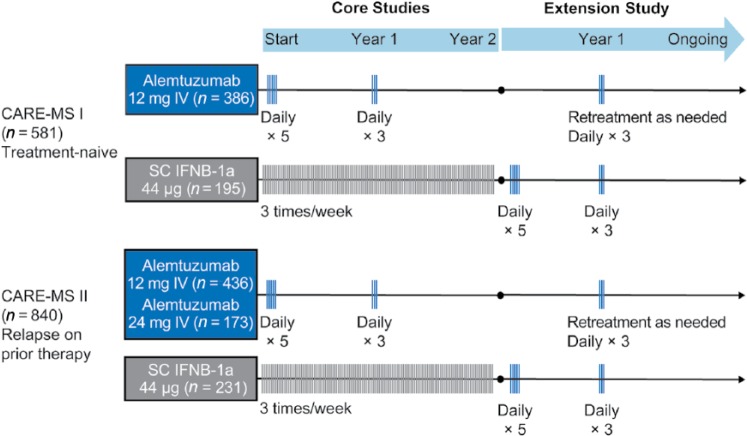
Figure 3.
CARE-MS phase III study design. CARE-MS studies were 2-year, phase III, randomized, active-controlled, head-to-head trials. Both studies enrolled patients with active relapsing-remitting multiple sclerosis (defined as ⩾2 relapses in the prior 2 years and ⩾1 relapse in the prior year). In the extension study, patients originally randomized to subcutaneous interferon beta-1a (SC IFNB-1a) received two annual courses of alemtuzumab 12 mg. Patients originally randomized to alemtuzumab could receive alemtuzumab retreatment if they fulfilled magnetic resonance imaging (MRI) or relapse criteria (⩾1 protocol-defined relapse in the previous year or ⩾2 unique MRI lesions on brain or spinal cord). Retreatment could occur at any point during the extension ⩾12 months after the previous treatment course. Only the 12-mg dose was used for retreatment.
CARE-MS, Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; IV, intravenous.