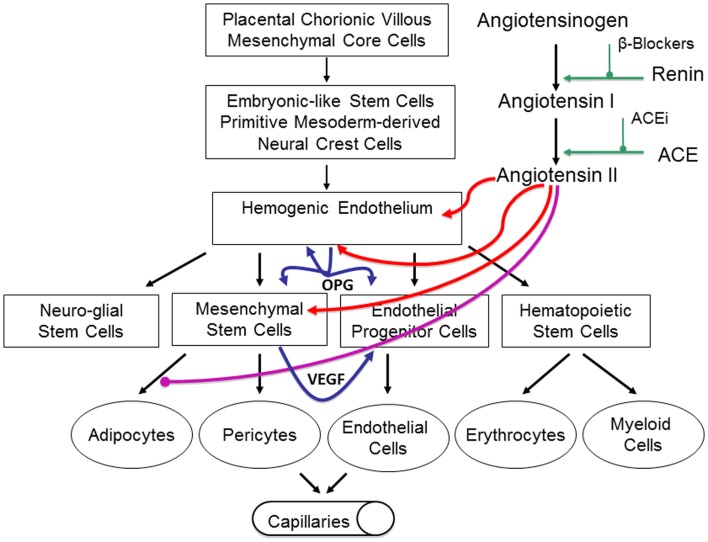
Figure 6.
Our proposed model of infantile hemangioma (IH) accounting for the observed programed biologic behavior and accelerated involution induced by modulators of the RAS, β-blockers, or ACE inhibitors. IH is caused by aberrantly displaced/embolized placental chorionic villous mensenchymal core cells into the fetus proper, which gives rise to a primitive mesoderm-derived hemogenic endothelium with a neural crest phenotype regulated by the RAS. This hemogenic endothelium differentiates into stem cells of neuro-glial, mesenchymal, endothelial, and hematopoietic lineages with downstream mesenchymal and erythropoetic and potentially myeloid differentiation capabilities. During the proliferative phase of IH, high levels of renin indirectly lead to high levels of ATII resulting in aberrant proliferation of the hemogenic endothelium and secretion of vascular endothelial growth factor (VEGF) from the accumulating mesenchymal stem cells (MSCs), both leading to proliferation of the endothelial progenitor cells (EPCs) and downstream endothelial cells (ECs). High levels of ATII also lead to over-expression of the TRAIL decoy receptor, osteoprotogerin (OPG) preventing apoptosis of the hemogenic endothelium, MSCs, and EPCs, with further proliferation and accumulation of these cellular elements and ECs. High levels of ATII also prevent terminal differentiation of MSCs to downstream adipocytes, further increasing the accumulation of MSCs. During the involuting phase of IH, reduced levels of ATII indirectly caused by decreasing levels of renin, ease accumulation of EPCs and ECs. Reduced levels of ATII also allow termination differentiation of MSCs into adipocytes resulting in a fibro-fatty residuum. Inhibition of renin by β-blockers or ACEi leads to reduced levels of ATII resulting in accelerated involution of IH. Reproduced with permission from Plastic and Reconstructive Surgery (67).