Figure 4.
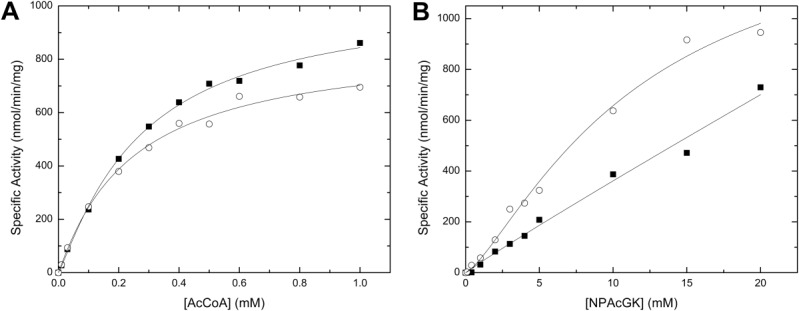
Effect of the His-tag on the kinetic activity of PA4794. A comparison of the kinetic curves for AcCoA (A) or NPAcGK (B) is shown for the enzyme with (filled squares) or without (open circles) a His-tag. Kinetic curves for AcCoA were performed using a set concentration of NPAcGK (10 mM) (A), and curves for NPAcGK were determined in the presence of 1 mM AcCoA (B). All kinetic assays were performed using 2.6 μM enzyme. The kinetic parameters for the PA4794 enzyme for AcCoA were the following: Vmax (1040 ± 52 (SD) (His-tagged) and 865 ± 67 (SD) (untagged) nmol/min/mg), S0.5 (272 ± 29 (SD) (His-tagged), and 244 ± 44 (SD) (untagged) μM). The kinetic parameters for the untagged PA4794 enzyme for NPAcGK were: Vmax (1460 ± 282 (SD) nmol/min/mg) and S0.5 (11.6 ± 3.8 (SD) mM). The NPAcGK curve did not reach saturation for the His-tagged enzyme, so we were unable to accurately determine its kinetic parameters.
