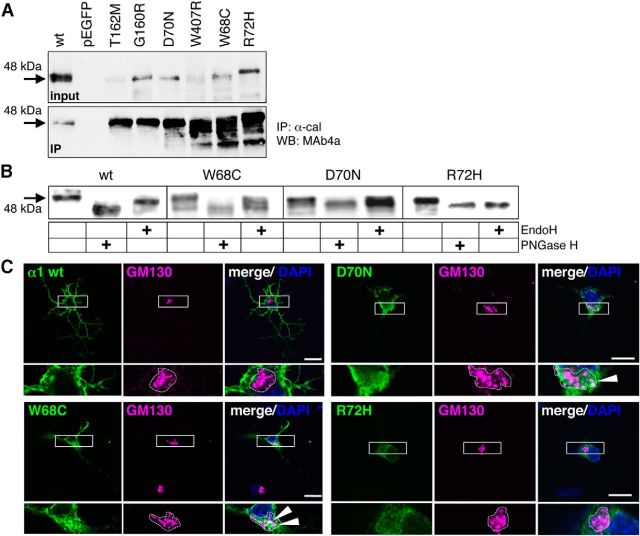
Figure 9.
GlyRα1 variants circumvent the ER quality control system. A, Immunoprecipiation of GlyR α1 loop D/β2–3 variants with anti-calnexin antibody stained for GlyRα1 with MAb4a (1:500). Input and IP are shown. Black arrows point to the appropriate molecular weight of GlyR α1 of 48 kDa. B, Analysis of the glycosylation pattern of loop D/β2–3 variants compared with WT. Endoglycosidases EndoH and PNGaseF were used for deglycosylation of α1 proteins and stained with MAb4a. Arrow points to the appropriate molecular weight of 48 kDa for glycosylated GlyR α1, 45 kDa of deglycosylated protein. Note the differences in the glycosylation pattern of R72H and W407R compared with WT and the other loop D/β2–3 mutants. C, Loop D/β2–3 variants transfected into hippocampal neurons. Images show GM130 costainings. White boxes mark the enlarged areas at higher magnification (bottom).