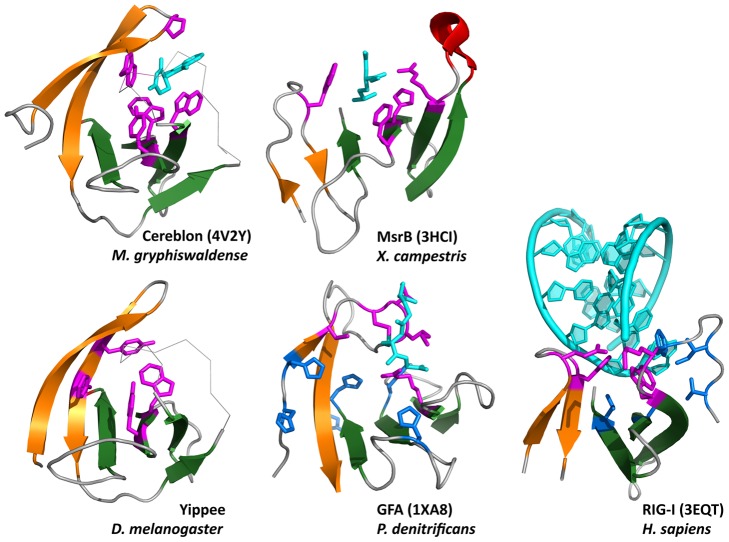
Figure 7. Gallery of β-tent domain binding sites.
The panels show the β-hairpin inserted between β2 and β3, and the C-terminal β-meander (β4–β7), colored as in Fig. 6. Residues involved in ligand binding are colored magenta and blue, and the ligands cyan. Yippee is the molecular model from Fig. S1 and the ligand-binding residues are predicted based on conservation and location in the fold. For GFA, only the residues involved in binding the glutatione cofactor are known (magenta). Six highly conserved histidine residues (blue) may participate in catalysis, but the exact location and geometry of the formaldehyde-binding site is unknown. For Rig-I, the residues involved in coordinating the RNA 3′ end are shown in magenta and the 5′ end in blue. Residue numbers are: Cereblon: P51, W79, W85, W99, Y101, Yippee: Y43, F45, W82, Y84, MsrB: W73, R97, H111, F113, GFA: C54, T57, L58, C56, R98 (magenta) and H32, H50, H52, H107, H117, H126 (blue), RIG-I: E573, H576, W604, K602 (magenta) and V632, L621, V595, I597, F601, W604 (blue).