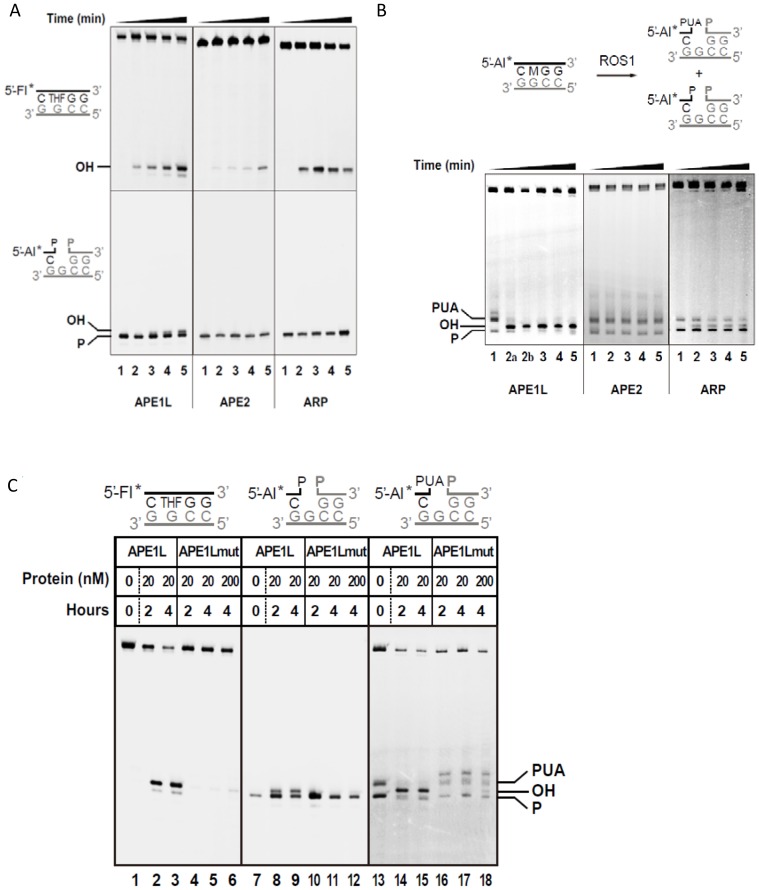
Figure 1. DNA repair activities of APE1L, APE2 and ARP.
(A) The AP endonuclease (upper panels) and 3′-phosphatase activities (lower panels) were tested by incubating proteins (20 nM) with DNA duplexes (40 nM) containing either a synthetic AP site (tetrahydrofuran, THF) opposite G, or a single-nucleotide gap flanked by 3′-phosphate and 5′-phosphate termini, respectively. Reactions were stopped at 0, 15, 30, 60 and 120 minutes and products were separated using a 12% denaturing polyacrylamide gel and detected by fluorescence scanning. (B) Activity against ROS1 products. Purified His-ROS1 (20 nM) was incubated at 30°C for 4 h with a DNA duplex (20 nM) containing a 5-meC residue opposite G. Reaction products (which contained a mixture of β- and β, δ-elimination products with 3′-PUA and 3′-phosphate ends, respectively, lane 1), were purified and incubated with APE1L, APE2 or ARP (20 nM). Reactions were stopped at 0, 15, 30, 60 and 120 minutes and products were detected as described above. Reaction in lane 2a was performed for 15 min with 2 nM APE1L. (C) Mutation N224D drastically reduces the repair activities of APE1L. WT and mutant versions of APE1L (20 nM or 200 nM, as indicated) were incubated with DNA substrates containing, opposite G, either a THF (40 nM), a single-nucleotide gap flanked by 3′-phosphate and 5′-phosphate termini (40 nM), or a mixture of β- and β, δ-elimination products generated by His-ROS1 (20 nM). Reactions were stopped at 2 or 4 h, as indicated, and products were separated using a 12% denaturing polyacrylamide gel and detected by fluorescence scanning.