Key Points
OSU-T315 impedes AKT localization in lipid rafts.
OSU-T315 shows in vitro and in vivo therapeutic effects.
Abstract
Aberrant regulation of endogenous survival pathways plays a major role in progression of chronic lymphocytic leukemia (CLL). Signaling via conjugation of surface receptors within the tumor environmental niche activates survival and proliferation pathways in CLL. Of these, the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway appears to be pivotal to support CLL pathogenesis, and pharmacologic inhibitors targeting this axis have shown clinical activity. Here we investigate OSU-T315, a compound that disrupts the PI3K/AKT pathway in a novel manner. Dose-dependent selective cytotoxicity by OSU-T315 is noted in both CLL-derived cell lines and primary CLL cells relative to normal lymphocytes. In contrast to the highly successful Bruton's tyrosine kinase and PI3K inhibitors that inhibit B-cell receptor (BCR) signaling pathway at proximal kinases, OSU-T315 directly abrogates AKT activation by preventing translocation of AKT into lipid rafts without altering the activation of receptor-associated kinases. Through this mechanism, the agent triggers caspase-dependent apoptosis in CLL by suppressing BCR, CD49d, CD40, and Toll-like receptor 9-mediated AKT activation in an integrin-linked kinase-independent manner. In vivo, OSU-T315 attains pharmacologically active drug levels and significantly prolongs survival in the TCL1 mouse model. Together, our findings indicate a novel mechanism of action of OSU-T315 with potential therapeutic application in CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in adults and remains incurable despite the introduction of targeted agents. CLL also has an uncertain etiology,1,2 although current data support that CLL originates from antigen-experienced, postgerminal center B cells.3 CLL has multiple recurrent cytogenetic abnormalities including del(13q14.3), trisomy 12, del(11q22.3), and del(17p13.1), of which the latter 2 portend a more rapid disease progression and shorter survival from diagnosis.4 Approximately 60% to 65% of CLL cases exhibit somatic hypermutation in immunoglobulin heavy chain variable (IGHV) genes (M-CLL), whereas 35% to 40% of CLL cases are categorized with unmutated IGHV status (U-CLL), which is associated with poor prognosis.5,6 The U-CLL patient subset also has a high proportion of ZAP-70 expression,7 enhanced B-cell receptor (BCR) signaling, and a disproportionate number of del(11q22.3) and del(17p13.1) cases. Overall, identification of biological markers associated with clinical outcome facilitates the identification of therapies targeted toward aberrant signaling pathways.
The current initial therapy for CLL patients lacking del(17p13.1) typically includes fludarabine and cyclophosphamide plus rituximab for younger, fit patients,8 whereas for older or infirm patients, chlorambucil plus obinutuzumab9 is most appropriate. Patients with del(17p13.1) do not benefit in terms of progression-free survival and overall survival with chemoimmunotherapy.10,11 Despite chemoimmunotherapy prolonging survival, this treatment is not curative. A proposed reason that available CLL therapies are incompletely effective is the increased proliferation and acquisition of tumor cell resistance to apoptosis as a result of stimuli within microenvironment of lymphoid tissues. Following recent advances in our understanding of CLL disease biology, efforts have focused on antagonizing oncogenic signaling initiated from the tumor microenvironment.12 Key prosurvival signals in CLL include BCR activation,13-15 the tumor necrosis factor receptor family molecules CD40L, B-cell activating factor, and a proliferation-inducing ligand,16,17 and the chemokines C-C motif ligand (CCL)-3, CCL4,18 C–X–C motif ligand (CXCL)-12,19 and CXCL13,20 all of which augment downstream activation of protein kinase B (AKT) and/or extracellular signal-regulated kinase (ERK) signaling in CLL cells and contribute to CLL survival and proliferation.21 To date, the best success in targeting the pathways activated by these signals has been through the use of agents inhibiting proximal or distal BCR signaling, such as the phosphoinositide 3-kinase (PI3K) p110δ inhibitor idelalisib22 and the Bruton's tyrosine kinase (BTK) inhibitor ibrutinib.23 Despite a high frequency of durable partial responses with these agents in CLL patients, complete remissions are infrequent. Indeed, none of these agents sufficiently overcomes AKT and/or ERK signaling pathways concurrently in the presence of multiple survival stimuli. Agents that inhibit AKT and/or ERK pathways in a novel manner therefore represent an exciting strategy for this disease.
The activation of the PI3K/AKT pathway is initiated at the plasma membrane, where phosphatidylinositol (3,4,5) trisphosphate (PIP3) generated by PI3 kinase recruits AKT to the unique membrane compartments termed lipid rafts on interaction via Pleckstrin homology domains, leading to its subsequent phosphorylation and activation by phosphoinositide-dependent kinase-1 (PDK)-1/2.24 Recent studies show that these glycosphingolipid- and cholesterol-rich rafts serve as platforms for the initiation of a variety of signaling pathways.25 These include the PI3K/AKT,26 CD40L,27 and BCR28 signaling pathways, each of which are associated with CLL tumor cell survival and disease progression. Notably, disruption of lipid rafts by cholesterol sequestration using saponin, cholesterol depletion by methyl-β-cyclodextrin,29 or inhibition of cholesterol biosynthesis by simvastatin30 results in ablation of AKT phosphorylation and induces preferential cytotoxicity toward malignant cells.31 Likewise, investigations using alkyl-lysophospholipid analogs further support the antitumor potential of targeting lipid rafts in mantle cell lymphoma and CLL via the recruitment of Fas/CD95.32 Building on these promising results, we herein report that the novel agent OSU-T315, modified from the scaffold of the AKT binding site at the integrin-linked kinase (ILK), uses an analogous mechanism of antitumor activity in CLL by displacing AKT from lipid rafts.
ILK is a master regulator of intracellular signaling that controls cell proliferation, survival, migration, invasion, and angiogenesis.33 The crucial function of ILK in tumorigenesis involves activation of AKT via Ser473 phosphorylation, suggesting ILK as a promising target in malignancies that rely on the PI3K pathway.34 Similarly, overexpression of ILK in myoblasts results in enhanced ERK activation, supporting the involvement of ILK in the mitogen-activated protein kinase (MAPK) pathway.35 Recently, generation of the novel ILK inhibitor OSU-T315 was reported.36 Given the justification that targeted therapy directed at ILK would be effective in CLL through suppressing AKT and/or ERK activation, we pursued preclinical in vitro and in vivo studies to determine the activity of OSU-T315 toward primary CLL cells. Concurrently, we demonstrate that this agent caused disruption of PI3K and MAPK signaling promoted by BCR or integrin engagement, besides specific inhibition of PI3K axis mediated by CD40 and TLR9. Remarkably, we verify that these effects are not through inhibition of ILK as expected, but rather by the prevention of AKT recruitment to lipid rafts. This surprising finding introduces a novel mechanism to therapeutically target CLL.
Materials and methods
Reagents and antibodies
OSU-T315 was synthesized as previously described.36 OSU-arg was acquired from the National Cancer Institute (Bethesda, MD). Stock solutions were prepared in dimethylsulfoxide. The commercial sources of antibodies used are listed in the supplemental Methods on the Blood Web site.
CLL, normal B-cell, and T-cell isolation
Peripheral blood samples were obtained from CLL patients after written informed consent was provided on an The Ohio State University institutional review board-approved protocol. Research was conducted in accordance with the Declaration of Helsinki. Human CLL and normal B or T cells were isolated and cultured as previously described23 and specified in the supplemental Methods.
Cell lysis and immunoblot
Cells were lysed in M-PER Mammalian Protein Extraction Reagent (Pierce). Proteins were quantified by the BCA protein assay kit (Pierce) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The blots were probed with the appropriate primary and horseradish peroxidase-conjugated secondary antibodies and developed with chemiluminescent substrate (Pierce), followed by detection using X-ray film and quantification using ImageJ software.
Assessment of cell death
Cell viability was assessed using annexin-V and propidium iodide (PI) double staining followed by analysis on an EPICS XL flow cytometer (Beckman-Coulter). Mitochondrial activity was measured by the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s instructions.
Animal studies
CD19+ cells were obtained from spleens of TCL1 transgenic mice with CLL-like leukemia and splenomegaly, and 1 × 106 cells were injected intravenously into a C57BL/6 mouse as previously described37 and specified in the supplemental Methods.
Statistical analysis
Percent viability of CLL compared with B or T cells on treatment was outlined by means with standard error of the mean and analyzed based on unpaired Student t tests. The ratios of phospho-protein were measured after normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. Differences between treatments were calculated by unpaired Student t tests. Overlap index of AKT content with lipid rafts was analyzed using Olympus FluoView viewer. For mouse models, overall survival was obtained using the Kaplan-Meier method. All tests were 2 sided, and statistical significance was declared for P < .05.
Results
OSU-T315 induces selective cytotoxicity toward CLL cells
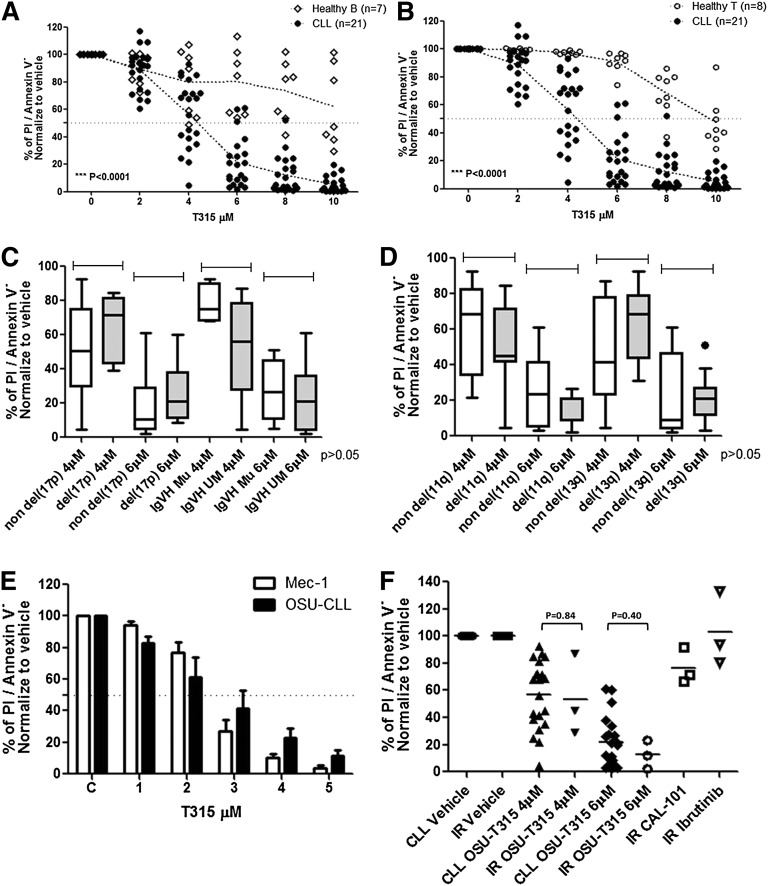
We first sought to assess the preclinical efficacy of OSU-T315 toward primary CLL cells. Our data indicate dose-dependent cytotoxicity toward CLL cells. The LC50 after 24-hour incubation of primary CLL (n = 21; Table 1) cells was 4.83 ± 0.83 μM. Contrasting with this, the cytotoxicity of OSU-T315 in normal B (n = 7) or T cells (n = 8) was significantly lower (LC50 > 10 μM; P < .0001 for each; Figure 1A-B), suggesting a therapeutic index of OSU-T315. CLL acquiring genomic aberrations including high-risk features del(17p13.1), unmutated IGVH, or del(13q14.3) and del(11q22.3) also reveal undifferentiated sensitivity toward OSU-T315 (Figure 1C-D). We next examined the effect of T315 toward 2 CLL-derived cell lines, Mec-1 and OSU-CLL (Figure 1E), and found similar dose-dependent cytotoxicity (LC50 of 2-3 μM in both) after 24-hour treatment. Remarkably, OSU-T315 exhibits comparable efficacy toward CLL cells from ibrutinib-resistant patients (Figure 1F).
Table 1.
CLL patients
| ID | 13q14.3 | 11q22.3 | 17p13.1 | trisomy 12 | IgVH |
|---|---|---|---|---|---|
| CLL 0083 | V | — | — | — | M 7.6% |
| CLL 0141 | V | — | — | — | M 2.1% |
| CLL 1330 | V | — | — | — | U 1.0% |
| CLL 1227 | V | V | — | — | U 0% |
| CLL 0588 | — | V | — | — | U 0% |
| CLL 0801 | V | V | — | — | U 0% |
| CLL 1201 | — | V | — | — | U 0% |
| CLL 0810 | — | — | — | — | U 0% |
| CLL 1529 | V | — | — | — | M 8.8% |
| CLL 1600 | V | — | — | — | U 0% |
| CLL 0881 | — | V | — | V | N/A |
| CLL 1461 | — | — | — | — | U 0.7% |
| CLL 0166 | — | — | — | — | U 0% |
| CLL 0742 | — | — | — | V | U 0% |
| CLL 1258 | — | — | V | — | U 0.7% |
| CLL 1766 | V | V | V | — | N/A |
| CLL 1593 | V | — | V | — | N/A |
| CLL 1446 | — | V | V | V | N/A |
| CLL 0170 | V | — | V | — | M 10% |
| CLL 0222 | V | — | V | — | U 0% |
| CLL 1606 | V | — | V | — | U 0.3% |
—, negative; M, mutated; N/A, not applicable; U, unmutated; V, positive.
Figure 1.
OSU-T315 induces preferential cytotoxicity in CLL cells. 1E7/mL primary CLL cells from patients; (A) healthy B cells (CD19+) and (B) T cells (CD3+) from leukopaks purified by the CD19+ or CD3+ enrichment kit, respectively, were incubated in complete RPMI with 10% fetal bovine serum followed by increasing dose of OSU-T315 treatment. Cells viability was analyzed by flow cytometry at 24 hours. (C) The response toward OSU-T315 in CLL with cytogenetic abnormalities were analyzed by del(17p13.1) and IGVH status or (D) del(11q22.3) and del(13q14.3). (E) 1E6/mL cells in complete RPMI with 10% fetal bovine serum was incubated and treated with increasing concentration of OSU-T315. Cells viability on treatment in Mec-1 or OSU-CLL cells was examined at 24 hours by Annexin V/PI staining. (F) Three ibrutinib-resistant samples were treated with OSU-T315 for 24 hours in comparison with nonresistant CLL cells; 1 μM ibrutinib was used for treatment for 30 minutes and then washed out; 1 μM CAL-101 was used for treatment for 24 hours.
OSU-T315 reduces Mcl-1 and Bcl-xl levels to trigger caspase activation in CLL cells
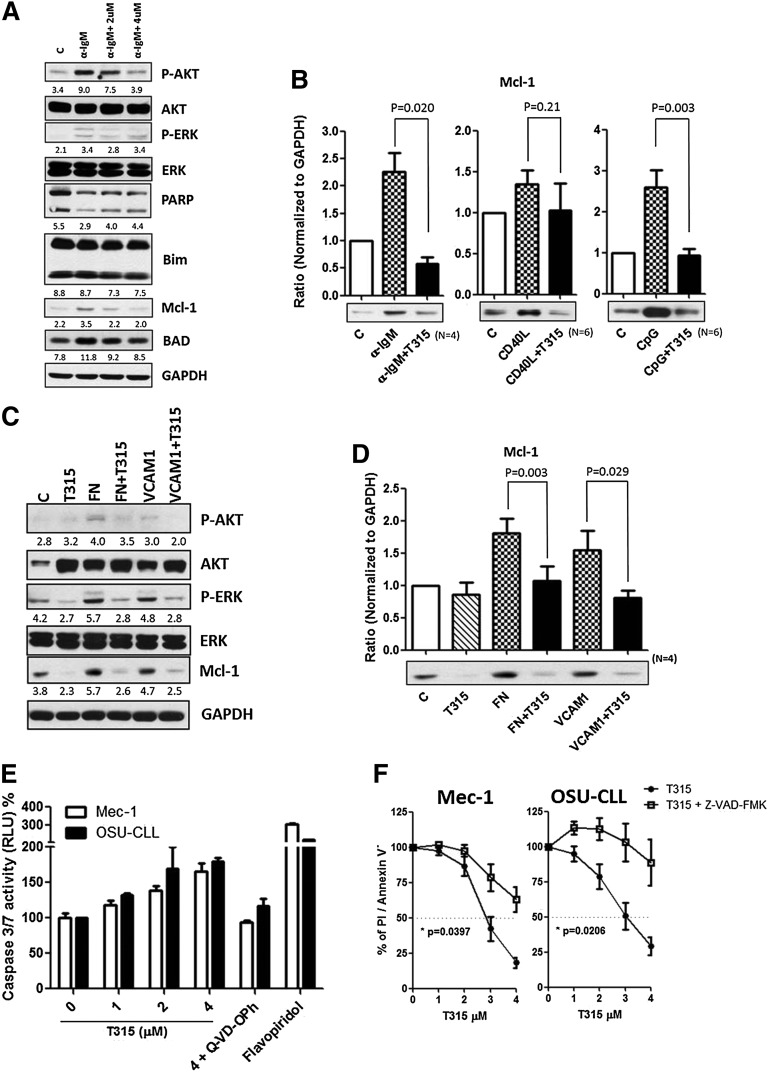
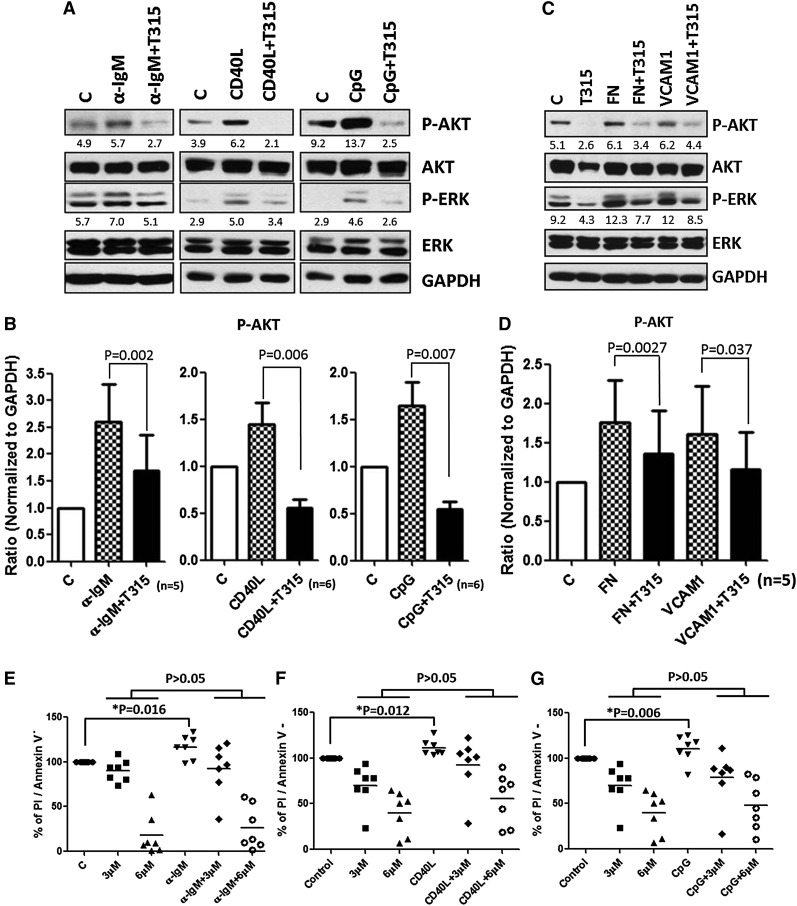
Bcl-2 family proteins are known to modulate apoptosis in CLL. Among these family members, Mcl-121,38 appears to play a particularly important role in CLL cell survival and can be upregulated by BCR activation.39 To characterize the effect of OSU-T315 on Bcl-2 family proteins, protein expression of Mcl-1, Bcl-2, Bad, and Bim was examined. Although expression levels of Bcl-2, Bad, and Bim were unaffected, Mcl-1 levels were increased upon BCR, TLR9, and CD49d activation, and OSU-T315 treatment was able to completely reverse this effect (Figure 2A-D). Remarkably, Mcl-1-overexpressing cells, which show resistance to fludarabine, were similarly sensitive to OSU-T315, indicating that OSU-T315 activity appears to be independent of Mcl-1, unlike the majority of currently available CLL agents (supplemental Figure 3A). Interestingly, Bcl-xl upregulation mediated by cytosine guanine dinucleotide (CpG) or CD40L was strongly repressed by OSU-T315 (supplemental Figure 3A). Because Mcl-1 or Bcl-xl is involved in antagonizing apoptosis in CLL,21,39 our results further suggest that caspase 3/7 activity is significantly augmented when increasing concentrations of OSU-T315 are administrated (Figure 2E). The importance of caspase activation in OSU-T315-mediated apoptosis was verified using Z-VAD-FMK, a pan-caspase inhibitor (Figure 2F). These data demonstrate that OSU-T315 mediates cytotoxicity in CLL or CLL-like cell lines in a caspase-dependent manner independent of Mcl-1 protein expression.
Figure 2.
The apoptotic machineries were induced on T315 treatment. (A) CLL cells were incubated in α-IgM coated plates or (C) fibronectin-coated plates or 1 μg/mL VCAM-1 with vehicle or OSU-T315 for 16 hours. Cell lysate was analyzed by immunoblotting and normalized to GAPDH, and the ratio of densitometry to GAPDH was shown beneath the blots. (B) Mcl-1 expression level was quantified according to the results of immunoblotting in the separated conditions of plate-coated α-IgM, 0.5 μg/mL CD40L, 3.2 μM CpG-ODN, or (D) fibronectin-coated plates or 1 μg/mL VCAM-1 along with the treatment of vehicle or 4 μM OSU-T315 for 16 hours. (E) Caspase 3/7 activity was accessed by Caspase-Glo 3/7 Assay Systems (Promega) and normalized to vehicle after 16-hour treatment; 20 μM of Q-VD-OPh served as the negative control, whereas 500 nM flavopiridol served as the positive control. (F) Mec-1 or OSU-CLL cells pretreated with vehicle or 20 μM z-VAD-FMK prior to 4 μM OSU-T315 treatment were analyzed by Annexin V/PI staining after 24 hours.
OSU-T315 inhibits AKT signaling independent of ILK
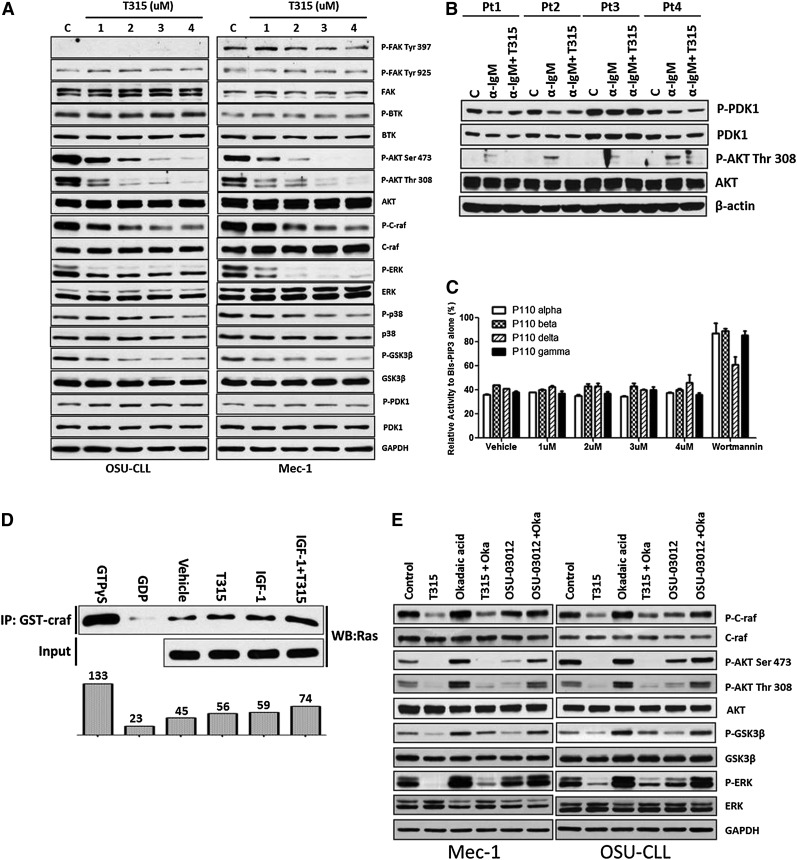
Activation of the PI3K/AKT pathway is essential for CLL survival and proliferation.21 Given that OSU-T315 has been shown to inhibit activation of AKT and ERK through targeting ILK in solid tumors,36 components of key signaling pathways were evaluated following OSU-T315 treatment in Mec-1 and OSU-CLL cells (Figure 3A and Table 2). Consistent with the previous report, this cellular profiling demonstrates notably diminished AKT and ERK phosphorylation on treatment, along with dephosphorylation of glycogen synthase kinase-3 beta (GSK-3β), a direct downstream target of AKT.40 To elucidate the role of ILK in phosphorylating Ser473 site of AKT, we silenced ILK by short hairpin RNA. Despite efficient knockdown of ILK (supplemental Figure 1A), the proliferation rate in Mec-1 cells was not altered (supplemental Figure 1B). Furthermore, ILK-depleted cells showed no differences in phosphorylation of AKT Ser473 or ERK, either at baseline or following anti-IgM (supplemental Figure 1C) or fibronectin (supplemental Figure 1D) stimulation. ILK downregulation also did not abrogate OSU-T315–mediated killing (supplemental Figure 1E). Additionally, the interaction between AKT and ILK is not affected (supplemental Figure 1F). Together, these results suggest a cytotoxic role of OSU-T315 independent of ILK in lymphoid leukemia cells.
Figure 3.
OSU-T315 targets intrinsic AKT and ERK signals cascades in CLL cells. (A) Lysate from Mec-1 and OSU-CLL cells treated with serial concentrations of OSU-T315 for 15 minutes are subjected to western blot analysis. (B) Lysate from primary CLL cells treated with OSU-T315 were subjected to analyze PDK1 (Ser241) and subsequent AKT (Thr308) activation. (C) In vitro kinase activity of class I PI3K is evaluated by the PI3 Kinase Activity/Inhibitor Assay Kit (Millipore) according to the instruction manual; 100 nM Wortmannin was applied as positive control. The biotinylated-PIP3 was set as 100%. The kinase reactions with vehicle or OSU-T315 were referenced to the biotinylated-PIP3 signal to have the relative percentage of inhibition. (D) RAS activity in 697 cells on treatments was measured by the Active Ras Detection Kit (Cell Signaling) according to the instruction manual. Guanosine triphosphate γS (positive control) and guanosine diphosphate (negative control) ensured the immunoprecipitation procedures worked properly, whereas insulin-like growth factor-1 served as a positive control to activate Ras. (E) Mec-1 and OSU-CLL cells pretreated with Okadaic acid (1 μM) were incubated with either OSU-T315 (4 μM) or OSU-03012 (5 μM), the PDK1 inhibitor, and the total lysate was subjected to immunoblotting to verify downstream signaling.
Table 2.
Densitometry for western blots for Figure 3A
| Mec-1 | OSU-CLL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | 1 μM | 2 μM | 3 μM | 4 μM | C | 1 μM | 2 μM | 3 μM | 4 μM | |
| P-FAKTyr397 | 6.5 | 8.2 | 6.2 | 5.1 | 5.0 | 2.2 | 1.9 | 2.4 | 2.6 | 2.4 |
| P-FAKTyr925 | 4.2 | 3.6 | 4.7 | 4.0 | 3.5 | 2.8 | 3.5 | 4.3 | 3.7 | 3.2 |
| P-BTK | 5.0 | 6.2 | 6.2 | 5.8 | 5.7 | 7.5 | 7.7 | 9.1 | 9.1 | 8.5 |
| P-AKTSer473 | 11.1 | 6.5 | 3.2 | 1.9 | 1.8 | 15.1 | 10.0 | 6.6 | 3.1 | 2.3 |
| P-FAKThr308 | 13.6 | 6.9 | 7.1 | 3.7 | 2.8 | 14.3 | 6.5 | 3.6 | 3.3 | 2.4 |
| P-Craf | 17.6 | 16.7 | 11.7 | 8.1 | 6.4 | 14.0 | 9.6 | 8.4 | 6.4 | 6.2 |
| P-ERK | 15.9 | 7.5 | 2.9 | 2.6 | 2.6 | 12.6 | 6.5 | 7.7 | 7.4 | 7.5 |
| P-P38 | 8.4 | 8.1 | 7.1 | 5.7 | 4.5 | 5.6 | 6.4 | 7.0 | 5.8 | 4.1 |
| P-GSK3β | 6.3 | 5.2 | 4.8 | 4.1 | 3.0 | 6.9 | 4.9 | 3.9 | 3.3 | 3.6 |
| P-PDK1 | 4.3 | 5.0 | 5.4 | 5.0 | 4.3 | 4.3 | 5.0 | 6.6 | 5.8 | 5.2 |
OSU-T315 does not influence proximal membrane signaling of AKT
Given that the localization of PDK1 to plasma membrane is essential to phosphorylate AKT and the generation of PIP3 via PI3-kinase is essential for this recruitment, we hypothesized that OSU-T315 may interfere with PDK1 activity or even proximal PI3K. To investigate these possibilities, we examined the effects of OSU-T315 on AKT-related signaling events. OSU-T315 treatment failed to disrupt PDK1 phosphorylation41 (Figure 3B and Table 3), PI3 kinase activity (Figure 3C), or Ras activity regarding the interaction with proto-oncogene serine/threonine-protein kinase (Figure 3D) under conditions in which AKT phosphorylation is inhibited. Other membrane-associated kinases including focal adhesion kinase (FAK) and BTK, a key component of the BCR signaling pathway, were also not affected by OSU-T315 (Figure 3A). Similarly, the activation of phosphatase PP2A, which has been known to negatively regulate AKT activity, was not influenced by T315 as evidenced by continued dephosphorylation of AKT in the presence of okadaic acid, the PP2A inhibitor (Figure 3E and Table 4). Together, these findings indicate that OSU-T315 induces cytotoxicity in CLL cells by suppressing intrinsic AKT activation without influencing proximal kinases or relevant phosphatases.
Table 3.
Densitometry for western blots for Figure 3B
| PT1 | PT2 | PT3 | PT4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | α-IgM | α-IgM+T315 | C | α-IgM | α-IgM+T315 | C | α-IgM | α-IgM+T315 | C | α-IgM | α-IgM+T315 | |
| P-PDK1 | 5.8 | 4.2 | 5.0 | 6.8 | 5.1 | 5.6 | 9.2 | 8.2 | 8.1 | 5.7 | 4.4 | 4.3 |
| P-AKTThr308 | 2.0 | 3.3 | 2.1 | 2.4 | 5.1 | 2.1 | 2.1 | 7.2 | 2.0 | 2.5 | 5.9 | 3.6 |
Table 4.
Densitometry for western blots for Figure 3E
| Mec-1 | OSU-CLL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | T315 | Okadaic acid | T315+Oka | OSU-03012 | OSU-03012+Oka | C | T315 | Okadaic acid | T315+Oka | OSU-03012 | OSU-03012+Oka | |
| P-Craf | 12.8 | 5.9 | 15.0 | 7.0 | 10.5 | 11.9 | 10.4 | 5.8 | 12.7 | 6.6 | 7.1 | 9.3 |
| P-AKTSer473 | 6.0 | 3.1 | 8.9 | 3.1 | 3.9 | 6.6 | 9.7 | 3.3 | 10.0 | 3.1 | 6.8 | 8.9 |
| P-FAKThr308 | 8.7 | 5.7 | 12.6 | 6.3 | 6.2 | 10.6 | 8.1 | 5.6 | 11.4 | 5.6 | 6.5 | 9.8 |
| P-GSK3β | 6.1 | 4.4 | 8.3 | 6.4 | 4.8 | 7.7 | 4.1 | 2.7 | 8.4 | 5.3 | 4.1 | 7.0 |
| P-ERK | 15.9 | 3.4 | 21.5 | 7.0 | 15.8 | 20.0 | 14.2 | 5.5 | 19.6 | 8.1 | 10.8 | 16.9 |
OSU-T315 antagonizes AKT membrane localization
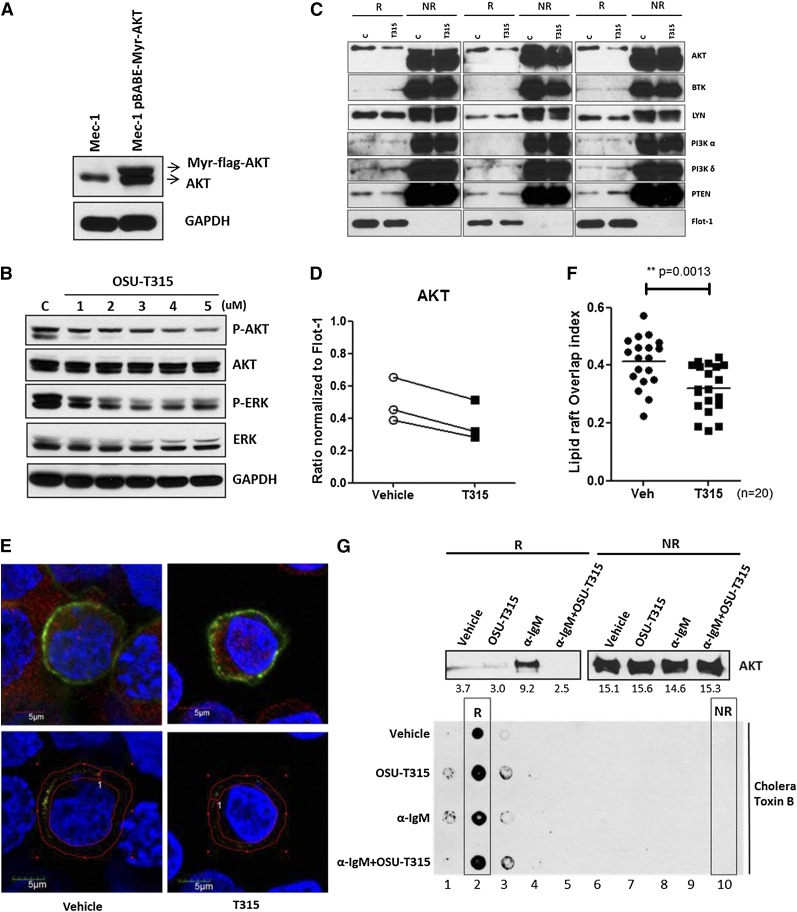
It is now evident that lipid rafts are highly important for the interactions and regulations of membrane-localized kinases, crucially mediating AKT translocation and downstream activation.30,31 As OSU-T315 inhibits AKT phosphorylation without affecting the activity of PI3K, PDK1, RAS, or PP2A, we postulated that OSU-T315 may elicit its effects through altering recruitment of AKT to lipid rafts. To determine whether OSU-T315 impedes the recruitment of AKT, myristoylated-AKT (myr-AKT) that spontaneously translocates to lipid rafts42 was introduced into Mec-1 cells to reinforce membrane docking of AKT in resting cells (Figure 4A). The diminished phosphorylation of myr-AKT was observed following OSU-T315 treatment (Figure 4B and Table 5), in parallel with reduced content of myr-AKT within the lipid raft fraction (Figure 4C-D and Table 6) while retaining equivalent amounts of PI3 kinases, phosphatase and tensin homolog (PTEN), and BCR-associated molecules Lyn and BTK. These data demonstrate that OSU-T315 inhibits AKT translocation into lipid rafts without affecting raft integrity. Furthermore, confocal microscopy to examine protein distribution shows reduced colocalization of myr-AKT with lipid rafts on OSU-T315 treatment (Figure 4E-F). Consistently, α-IgM-induced AKT translocation is abrogated by OSU-T315 (Figure 4G). Together, these findings confirm that OSU-T315 inhibits AKT activation by preventing its localization into lipid rafts.
Figure 4.
OSU-T315 impairs AKT translocation to lipid raft subdomains in plasma membrane. (A) Mec-1 cells were retro-virally transduced with pBABE-Myr-flag-AKT vector (Addgene). The total lysates were subjected to western blot to verify the myristoylated AKT expression. (B) Mec-1 expressing Myr-flag-AKT was treated with increasing concentration of OSU-T315. Total lysate was analyzed by immunoblotting. (C) Lipid raft from Mec-1 cells expressing Myr-flag-AKT was purified after vehicle or OSU-T315 (4 μM) treatment by the ultracentrifugation approach. The raft (R) and nonraft (NR) fractions were analyzed for raft-associated molecules, and Flotillin-1 serves as a lipid raft marker. (D) The 3 independent studies were quantified for AKT content in raft compartment. (E) Mec-1 cells with Myr-flag-AKT were subject to immunofluorescence staining with antibodies of α-AKT (Alexa 594) and α- Cholera toxin subunit B (CT-B) (Alexa 488). (F) The colocalization index was measured and analyzed by confocal microscope double blindly. (G) 697 cells were treated with plate-bounded α-IgM, 4 μM OSU-T315, or in combination for 1 hour, and the lipid raft fractions were extracted for analysis. Cholera toxin subunit B (CT-B) is the marker for lipid raft compartment.
Table 5.
Densitometry for western blots for Figure 4B
| C | 1 μM | 2 μM | 3 μM | 4 μM | 5 μM | |
|---|---|---|---|---|---|---|
| P-AKT | 6.1 | 3.7 | 3.6 | 3.6 | 3.3 | 3.1 |
| P-ERK | 9.7 | 6.5 | 5.6 | 4.8 | 5.5 | 6.7 |
Table 6.
Densitometry for western blots for Figure 4C
| C | T315 | C | T315 | C | T315 | |
|---|---|---|---|---|---|---|
| AKT | 5.1 | 3.9 | 7.5 | 5.5 | 4.7 | 3.1 |
| BTK | 2.2 | 4.1 | 4.8 | 5.6 | 3.3 | 3.8 |
| LYN | 8.6 | 9.7 | 5.6 | 6.7 | 6.1 | 5.1 |
| P13K α | 5.7 | 5.0 | 4.9 | 5.2 | 3.3 | 4.1 |
| P13K δ | 8.3 | 10.6 | 8.0 | 7.4 | 2.7 | 5.4 |
| PTEN | 5.2 | 6.5 | 6.5 | 6.9 | 3.8 | 5.5 |
BCR-, CD40-, and Toll-like receptor 9-induced survival signals are abrogated by OSU-T315
Diverse stimuli within tumor environments provoke ATK activation. Numerous studies indicate that the BCR is a key transmitter of survival signals in CLL cells.14 On engagement of BCR, PI3K and MAPK cascades are activated, from which CLL acquires the competency to evade apoptosis. To assess whether OSU-T315 can overcome external survival stimuli, CLL cells stimulated with immobilized α-IgM, CD40L, or CpG were treated with different concentrations of OSU-T315. The results suggest the potency of OSU-T315 suppressing downstream AKT activation triggered by external factors (Figure 5A-B). The protection of CLL cells mediated by these external stimuli was abrogated by increasing doses of OSU-T315 (Figure 5E-G).
Figure 5.
OSU-T315 inhibits BCR-, CD40L-, and CpG-induced survival signal. (A) CLL cells were treated with either plate-bounded α-IgM, 0.5 μg/ml CD40L, 3.2 μM CpG-ODN, or (C) plate-bound fibronectin or 1 μg/ml VCAM-1 combined with vehicle or 4 μM OSU-T315 for 15 minutes. The total lysate was subjected to immunoblotting. (B,D) Data from individual patients were quantified for p-AKT level and normalized to GAPDH. (E-G) Cell viability was examined after 24 hours in the treated conditions of plate-bounded α-IgM, 0.5 μg/ml CD40L, or 3.2 μM CpG-ODN combined with vehicle or different doses of OSU-T315.
CD49d-mediated survival signals are abolished by OSU-T315
CLL cells traffic and interact with stromal or nurse-like cells within lymphoid tissues through the VLA-4 (α4β1, CD49d/CD29) complex, from which survival signals originate to prevent spontaneous or drug-induced apoptosis in part resulting from AKT activation.43,44 Given that OSU-T315 interferes with integrin signaling as evidenced by inhibition of fibronectin-induced AKT phosphorylation, we examined the potential of OSU-T315 to block CD49d-mediated protection of CLL cells. Our results demonstrate CD49d activation via interaction with fibronectin or vascular cell adhesion molecule (VCAM)-1 can promote both AKT and ERK signals, and these effects are suppressed by OSU-T315 (Figure 5C-D).
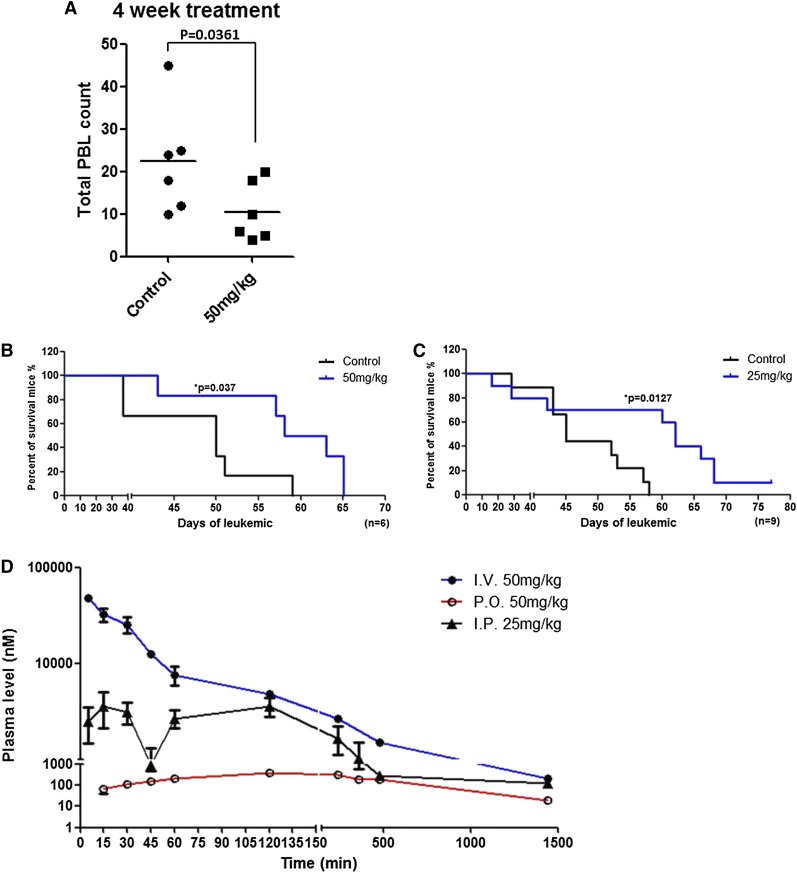
OSU-T315 has in vivo activity in the TCL1 mouse model of human CLL
Eμ-TCL1 adult mice develop clonal B-cell leukemia with surface expression of immunoglobulin (IgM)+/CD19+/CD5+,45 exhibiting features similar to human CLL cells with regard to activation of AKT signaling, in part resulting from the interaction of TCL1 with AKT and subsequent enhancement of its kinase activity.46 Thus, leukemia cells derived from Eu-TCL1 mice were transplanted into C57BL/6 mice to assess the in vivo efficacy of OSU-T315. Mice that developed leukemia in the bloodstream (≥10% CD5+/CD19+ cells) were treated orally with vehicle or OSU-T315 (50 mg/kg daily). Mice that received OSU-T315 show significantly reduced white blood cell (WBC) counts after 4 weeks of treatment (P = .036; n = 6 per group; Figure 6A). This improvement also correlated with prolonged overall survival in the OSU-T315-treated group (P = .037; n = 6 per group; Figure 6B). Improved in vivo efficacy of OSU-T315 was further validated by intraperitoneal delivery approach (P = .012; n = 9 per group; Figure 6C). Collectively, these findings show that OSU-T315 displays the capacity to delay leukemia progression and significantly increase overall survival in mice bearing TCL1 leukemia cells.
Figure 6.
Prolonged survival of TCL1 leukemia-engrafted mice by OSU-T315. (A) C57BL/6 mice engrafted with TCL1 leukemia cells were treated orally with vehicle or 50 mg/kg OSU-T315 daily after appearance of 10% leukemia cells in the peripheral blood. WBCs were monitored by blood smear slides weekly until euthanization was required. Data represent WBC counts after 4-week treatment. (B) Overall survival was analyzed after treatment starts (n = 6 per group). (C) C57BL/6 mice engrafted with TCL1 leukemia cells were treated by intraperitoneal injection with vehicle or 25 mg/kg OSU-T315 once daily for 2 weeks after the appearance of 5% CD19+, CD5+ leukemia cells among CD45+ peripheral blood mononuclear cells. OSU-T315 was formulated in phosphate-buffered saline containing 10% Cremophor EL (Sigma). After a 2-week daily scheme, leukemic mice were treated every other day with vehicle or 25 mg/kg OSU-T315 to prevent weight loss. The percent of leukemic cells was monitored weekly by flow cytometry until euthanization was required. Overall survival was analyzed (n = 9 for each group). (D) Data represent the pharmacokinetics of OSU-T315 in plasma after intravenous, intraperitoneal, or oral dosing (n = 6 per group).
Pharmacokinetic properties of OSU-T315
Previous studies verified the efficacy of orally administered OSU-T315 in mice xenografted with prostate cancer cells.36 Although our data also confirm the in vivo efficacy of OSU-T315 in leukemia, pharmacokinetic studies on OSU-T315 remain necessary to optimize the treatment regimen. Therefore, plasma from mice treated with OSU-T315 by oral gavage or intravenous or intraperitoneal injection was analyzed (Figure 6D and Table 7). Results indicate a similar elimination half-life by these delivery approaches. Although intravenous administration achieves peak concentration of OSU-T315 within 5 minutes, either intraperitoneal or oral administration requires 2 hours to reach maximum levels in systemic circulation, and these maximum levels are substantially lower than the value achieved via the intravenous route, which may relate to less favorable absorption, distribution, metabolism, and excretion properties of OSU-T315 in existing formulation. Importantly, the low bioavailability (5.8%) via the oral route may in part have contributed to the relatively modest survival benefit of this agent, and strategies are being explored to address this obstacle.
Table 7.
Pharmacokinetic properties of OSU-T315
| PK parameters | Intravenous (50 mg/kg) | Orally (50 mg/kg) | Intraperitoneally (25 mg/kg) |
|---|---|---|---|
| Cmax (nM) | 48 764 | 369.09 | 3604 |
| Tmax (minutes) | 5 | 120 | 120 |
| T1/2 (hours) | 5.51 | 5.07 | 6.08 |
| Bioavailability (%) | NA | 5.8 | 58.6 |
NA, not applicable.
Discussion
The data report herein characterize a novel mechanism of action of OSU-T315. Specifically, we demonstrate that OSU-T315 displaces AKT from lipid rafts, thus impairing AKT phosphorylation induced by a variety of pathways including BCR, CD40L, TLR9, and integrin. The downstream consequences include downregulation of Mcl-1 or Bcl-xl and caspase-dependent apoptosis. Remarkably, OSU-T315 demonstrates potential therapeutic implications in high-risk patients with del(17p13.1) or unmutated IGVH, as well as ibrutinib-resistant patients. We further clarify in vivo efficacy showing improved median survival in a TCL-1 transplant model, along with selective targeting of CLL cells relative to normal B or T lymphocytes.
Targeting BCR-associated kinases with small compounds has emerged as an exciting new treatment paradigm in CLL.47 The shift of focus toward BCR-associated kinases is owed in part to a better understanding of the essential role of the BCR in CLL pathogenesis and the promising clinical results achieved with continuous dosing of BTK and PI3K inhibitors. Notably, all agents effective in targeting BCR signaling suppress the PI3K/AKT signaling cascade. Unusual activation of AKT drives CLL expansion and evades apoptotic mechanisms, critically contributing to this lymphoproliferative disorder.48 Previous studies identified the importance of the PI3K δ subunit in provoking AKT activation in CLL cells, thus driving the development of Idelalisib (GS-1101)49 and IPI-145 (INK-1147)50 that both show significant clinical activity through abrogating the PI3K/AKT axis. Phosphorylation of AKT initially occurs at the Thr308 site in the activation loop by PDK1, with full activation occurring following phosphorylation of Ser473 in the C-terminal hydrophobic domain by the putative PDK2 kinase. In contrast to existing agents targeting upstream kinases, OSU-T315 impedes the recruitment of AKT to its signalosome in the lipid raft. The characteristics of OSU-T315 provide the following advantages. (1) Shortcomings of recent strategies in developing targeted therapeutic arise from the balancing between specificity and efficiency. As multiple stimuli lead to AKT activation, ubiquitously inhibition of this survival signal is challenging. The effect of OSU-T315 via AKT regardless of upstream pathways activity may improve efficacy. Contrary to current AKT inhibitors that abrogate phosphorylation to selective isoforms, OSU-T315 prevents overall phosphorylation of AKT by targeting raft translocation rather than showing selectivity toward a particular isoform. (2) The functional redundancy of different PI3Ks may result in the efficacy and drive drug resistance for long-term treatment. Compared with existing PI3K inhibitors in CLL clinical practice, OSU-T315 shows the potential to surpass the issue. (3) The preferential cytotoxicity of OSU-T315 toward CLL cells compared with normal B and T cells suggests a beneficial therapeutic window.
PI3K/AKT signaling originates from lipid rafts following the recruitment of AKT via the interaction of the PH domain of AKT with PIP3 or PIP2.30,31 In B cells, Src family kinases including Lyn, as well as the CD40 receptor, have been shown to be stably anchored within lipid rafts. Certain proteins, including AKT, are accumulated in rafts in part due to myristoylation. Our observations suggest that OSU-T315 impairs the ability of AKT to be retained in cholesterol-enriched lipid rafts as previously described,26 resembling the effect of cholesterol inhibitors such as simvastatin.30,31 This effect was also observed with synthetic antitumor lipids (ATLs). Consistent with the assertions that both ATLs51,52 and cholesterol inhibitors30 exhibit selective cytotoxicity toward malignant cells, a similar effect is observed in OSU-T315. These combined observations suggest that hypercholesterolemia might potentiate CLL development53 and also that our novel lipid raft-targeting pathway could show significant clinical activity in CLL. In contrast to ATLs that impact diverse molecules in lipid rafts such as PI3K, PDK1, and mechanistic target of rapamycin in addition to AKT,38 OSU-T315 appears to target AKT alone, indicating that this agent uniquely blocks the component essential for AKT docking. We are currently investigating the underlying candidates by OSU-T315 using proteomic approaches.
OSU-T315 was originally designed to specifically disrupt the interaction of AKT with its binding site on ILK, a putative PDK2 anticipated as a promising therapeutic target in several cancers. Studies assessing the function of ILK in CLL or other leukemias are lacking. Our data showed slightly lower amounts of ILK in CLL compared with normal cells (supplemental Figure 2A-B), and ILK knockdown could not recapitulate cytotoxicity by OSU-T315. Together, these data do not support a strong role for ILK in CLL progression. Besides being activated by phospholipase C-γ2/protein kinase C-β through the BCR pathway, ERK1/2 is also provoked via diverse external signals, leading to CLL proliferation and survival.54 Interestingly, despite the comprehensive profiling by immunoblotting showing reduction of tonic phospho-AKT and p-ERK on OSU-T315 treatment, only BCR- or CD49d-mediated ERK activation was consistently abrogated by OSU-T315. Conversely, CD40L- or CpG-induced signaling exhibited differential response in ERK status (supplemental Figure 4), suggesting that OSU-T315 exclusively suppressing PI3K/AKT signaling induced by diverse stimuli. Finally, our in vivo study shows that, despite being hampered by low oral bioavailability, OSU-T315 significantly prolonged survival of leukemic mice. These promising data support the development of improved formulation, delivery strategies, and/or derivatives, which are being actively pursued by our group. Together, these findings provide an alternative strategy to target CLL cell survival by disrupting AKT recruitment to lipid rafts and introduce an outstanding candidate for further investigation and development in CLL and potentially other B-cell malignancies.
Acknowledgments
This work was supported by the Specialized Center of Research from the Leukemia and Lymphoma Society, National Cancer Institute grants P50-CA140158, R01 CA177292, R01 CA183444, and P01 CA95426, the D. Warren Brown Foundation, the Four Winds Foundation, The Sullivan Chronic Lymphocytic Leukemia Research Fund, Mr and Mrs Michael Thomas, Al and Midge Lipkin, and the Harry Mangurian Foundation.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.-M.L. designed the research, performed experiments, analyzed data, generated figures, and wrote the manuscript; Y.L., J.A.W., K.B., and J.M. were involved in planning components of the research, performed experiments, reviewed drafts, and approved the final version of the paper; Y.-Y.Y. and E.H. were involved in planning components of the research, reviewed drafts, and approved the final version of the paper; X.Z. and A.L. performed statistical analysis; S.B.S. and C.-S.C. were involved in planning components of the research and provided necessary reagents essential to the hypothesis of this paper; F.A., J.A.J., L.A.A., and K.M. reviewed drafts and approved the final version of the paper; and M.A.P., J.C.B., and A.J.J. planned every aspect of the proposal, supervised the research, reviewed and modified drafts, obtained funding for the research work, and approved the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John C. Byrd, Rm 455B, 410 West 12th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu; or Amy J. Johnson, Rm 455C, 410 West 12th Ave, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.
References
- 1.Zenz T, Mertens D, Küppers R, Döhner H, Stilgenbauer S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10(1):37–50. doi: 10.1038/nrc2764. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 3.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 4.Zenz T, Mertens D, Döhner H, Stilgenbauer S. Importance of genetics in chronic lymphocytic leukemia. Blood Rev. 2011;25(3):131–137. doi: 10.1016/j.blre.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–1854. [PubMed] [Google Scholar]
- 6.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847. [PubMed] [Google Scholar]
- 7.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348(18):1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 8.Hallek M, Fischer K, Fingerle-Rowson G, et al. International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 9.Robak T. GA-101, a third-generation, humanized and glyco-engineered anti-CD20 mAb for the treatment of B-cell lymphoid malignancies. Curr Opin Investig Drugs. 2009;10(6):588–596. [PubMed] [Google Scholar]
- 10.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 11.Stephens DM, Ruppert AS, Jones JA, et al. Impact of targeted therapy on outcome of chronic lymphocytic leukemia patients with relapsed del(17p13.1) karyotype at a single center. Leukemia. 2014;28(6):1365–1368. doi: 10.1038/leu.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114(16):3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herishanu Y, Pérez-Galán P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dühren-von Minden M, Übelhart R, Schneider D, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489(7415):309–312. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 15.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–4475. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo T, Nishio M, Enzler T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood. 2007;109(2):703–710. doi: 10.1182/blood-2007-04-081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244(1):115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burger JA, Quiroga MP, Hartmann E, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113(13):3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Möhle R, Failenschmid C, Bautz F, Kanz L. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1). Leukemia. 1999;13(12):1954–1959. doi: 10.1038/sj.leu.2401602. [DOI] [PubMed] [Google Scholar]
- 20.Dürig J, Schmücker U, Dührsen U. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001;15(5):752–756. doi: 10.1038/sj.leu.2402107. [DOI] [PubMed] [Google Scholar]
- 21.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111(2):846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 22.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 26.Adam RM, Mukhopadhyay NK, Kim J, et al. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007;67(13):6238–6246. doi: 10.1158/0008-5472.CAN-07-0288. [DOI] [PubMed] [Google Scholar]
- 27.Pham LV, Tamayo AT, Yoshimura LC, et al. A CD40 Signalosome anchored in lipid rafts leads to constitutive activation of NF-kappaB and autonomous cell growth in B cell lymphomas. Immunity. 2002;16(1):37–50. doi: 10.1016/s1074-7613(01)00258-8. [DOI] [PubMed] [Google Scholar]
- 28.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2(2):96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 29.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110(5):597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115(4):959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–2231. [PubMed] [Google Scholar]
- 32.Mollinedo F, de la Iglesia-Vicente J, Gajate C, et al. In vitro and In vivo selective antitumor activity of Edelfosine against mantle cell lymphoma and chronic lymphocytic leukemia involving lipid rafts. Clin Cancer Res. 2010;16(7):2046–2054. doi: 10.1158/1078-0432.CCR-09-2456. [DOI] [PubMed] [Google Scholar]
- 33.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci. 2008;121(Pt 19):3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 34.Persad S, Attwell S, Gray V, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276(29):27462–27469. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Li J, Zhang Y, Wu C. The roles of integrin-linked kinase in the regulation of myogenic differentiation. J Cell Biol. 2000;150(4):861–872. doi: 10.1083/jcb.150.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SL, Hsu EC, Chou CC, et al. Identification and characterization of a novel integrin-linked kinase inhibitor. J Med Chem. 2011;54(18):6364–6374. doi: 10.1021/jm2007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207–1213. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smit LA, Hallaert DY, Spijker R, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109(4):1660–1668. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- 39.Petlickovski A, Laurenti L, Li X, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105(12):4820–4827. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 40.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 41.Casamayor A, Morrice NA, Alessi DR. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J. 1999;342(Pt 2):287–292. [PMC free article] [PubMed] [Google Scholar]
- 42.Kohn AD, Takeuchi F, Roth RA. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271(36):21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 43.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. [PubMed] [Google Scholar]
- 44.Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114(20):4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson AJ, Lucas DM, Muthusamy N, et al. Characterization of the TCL-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood. 2006;108(4):1334–1338. doi: 10.1182/blood-2005-12-011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pekarsky Y, Koval A, Hallas C, et al. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci USA. 2000;97(7):3028–3033. doi: 10.1073/pnas.040557697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robak P, Robak T. A targeted therapy for protein and lipid kinases in chronic lymphocytic leukemia. Curr Med Chem. 2012;19(31):5294–5318. doi: 10.2174/092986712803833371. [DOI] [PubMed] [Google Scholar]
- 48.Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol. 2011;23(2):178–183. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IPI-145 shows promise in CLL patients. Cancer Discovery. 2014;4(2):136. doi: 10.1158/2159-8290.CD-NB2013-177. [DOI] [PubMed] [Google Scholar]
- 51.Mollinedo F, Fernández-Luna JL, Gajate C, et al. Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (Edelfosine): molecular structure requirements, cellular uptake, and protection by Bcl-2 and Bcl-X(L). Cancer Res. 1997;57(7):1320–1328. [PubMed] [Google Scholar]
- 52.Gajate C, Mollinedo F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood. 2007;109(2):711–719. doi: 10.1182/blood-2006-04-016824. [DOI] [PubMed] [Google Scholar]
- 53.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122(6):1418–1421. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 54.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120(6):1175–1184. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]