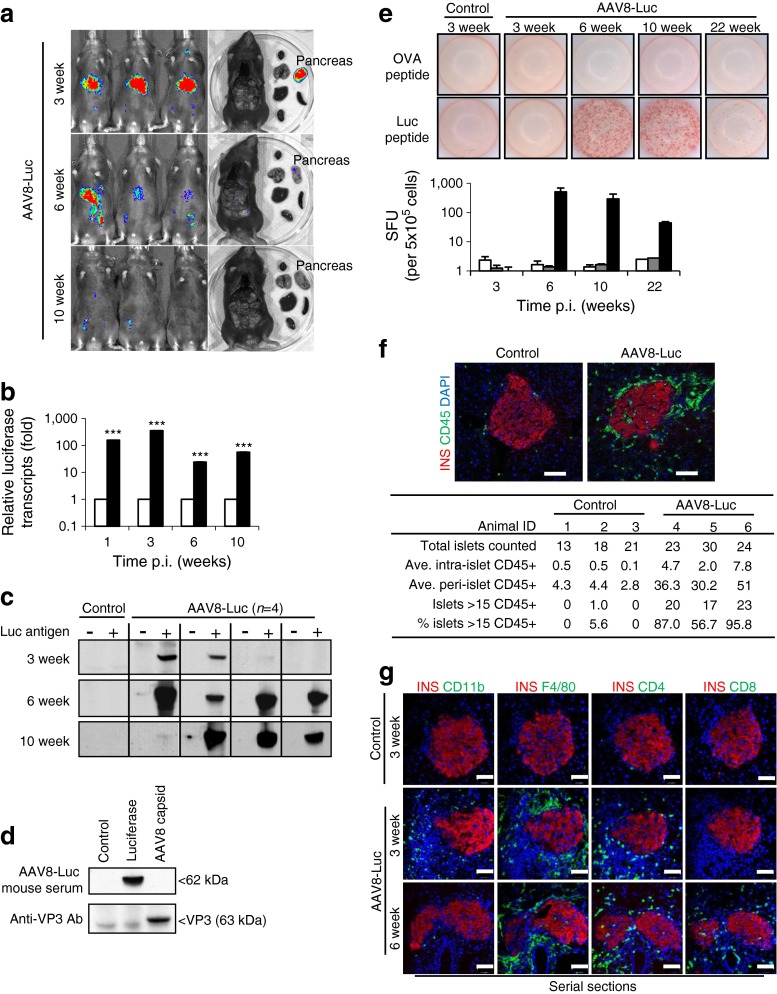
Fig. 2.
Beta cell-targeted luciferase expression induces specific humoral and cellular immune responses. (a) Luciferase expression was monitored 3, 6 and 10 weeks p.i. (b) RT-PCR analysis of total pancreatic lysates for firefly luciferase transcripts in saline controls and AAV8-Luc (white and black bars, respectively); data represents fold change vs age-matched controls set as 1; logarithmic scale, base 10; ***p < 0.001 vs controls. (c) Western blot detection of anti-luciferase antibodies in mouse plasma samples. (d) Lysates from 293T cells overexpressing luciferase and AAV8 capsid proteins and from uninfected controls were used as an antigen source to detect antibodies against AAV8 capsid. Plasma samples were from mice 6 weeks p.i. (n = 4). Anti-VP3 antibody was used as a control. (e) An ELISpot assay was performed to detect cytotoxic T cells from splenocytes of control (white bars) and AAV8-Luc (black bars) infected mice that react to the luciferase epitope peptide LMYRFEEEL. Ovalbumin (OVA) peptide was used as a negative control (grey bars). Splenocytes were analysed after 3, 6, 10 and 22 weeks. Representative wells and the means ± SEM of spot-forming units (SFU) from four mice per group per time point are shown. Logarithmic scale, log base 10. (f) Representative islets used for insulitis scoring with an anti-CD45 antibody at 4 weeks p.i. The table shows the number of random islets counted and the average (Ave.) intra- and peri-islet CD45 cells (n = 3 per group). (g) Serial pancreatic sections were stained with antibodies against the immune cell markers CD11b, F4/80, CD4 and CD8 (green). Nuclei were counterstained with DAPI (blue). Scale bars, 50 μm. INS, insulin; Luc, luciferase