Abstract
Androgen receptor gene (AR), monoamine oxidase A gene (MAOA) and monoamine oxidase B gene (MAOB) have been found to have associations with behavioral traits, such as aggressiveness, and disorders in humans. However, the extent to which similar genetic effects might influence the behavior of wild apes is unclear. We examined the loci AR glutamine repeat (ARQ), AR glycine repeat (ARG), MAOA intron 2 dinucleotide repeat (MAin2) and MAOB intron 2 dinucleotide repeat (MBin2) in 32 wild bonobos, Pan paniscus, and compared them with those of chimpanzees, Pan troglodytes, and humans. We found that bonobos were polymorphic on the four loci examined. Both loci MAin2 and MBin2 in bonobos showed a higher diversity than in chimpanzees. Because monoamine oxidase influences aggressiveness, the differences between the polymorphisms of MAin2 and MBin2 in bonobos and chimpanzees may be associated with the differences in aggression between the two species. In order to understand the evolution of these loci and AR, MAOA and MAOB in humans and non-human primates, it would be useful to conduct future studies focusing on the potential association between aggressiveness, and other personality traits, and polymorphisms documented in bonobos.
Abbreviations: AR, androgen receptor gene; MAOA, monoamine oxidase A gene; MAOB, monoamine oxidase B gene; ARQ, repeat locus coding glutamine in androgen receptor gene; ARG, repeat locus coding glycine in androgen receptor gene; MAin2, repeat locus in intron 2 of monoamine oxidase A gene; MBin2, repeat locus in intron 2 of monoamine oxidase B gene; PCR, polymerase chain reaction.
Keywords: Bonobo, Androgen receptor, Monoamine oxidase, Genetic variation
Introduction
Numerous studies have examined the association between neurotransmitter- or hormone-related gene polymorphisms and behavioral traits and disorders in humans (D'Souza and Craig, 2006, Ebstein, 2006). Accordingly, androgen receptor gene (AR), monoamine oxidase A gene (MAOA) and monoamine oxidase B gene (MAOB) are candidate genes.
Androgen receptor is a DNA-binding transcription factor, the main regulator of androgen signaling in the cell, activated mostly by testosterone and 5α-dihydrotestosterone. Androgen receptor is encoded by the X-chromosome-located AR, that has two polymorphic trinucleotide repeat regions — CAG encoding glutamine and GGN encoding glycine (ARQ and ARG, respectively) — on the N-terminal domain in the first exon in humans (Chang et al., 1988). ARQ and ARG have been found to be associated with several personality disorders (Aluja et al., 2011, Comings et al., 1999), prostate cancer (Zhai et al., 2014) and certain personality traits like aggression and dominance (Jönsson et al., 2001, Vermeersch et al., 2010), or neuroticism and extraversion (Westberg et al., 2009). In search of the reasons for the numerous phenotypic associations of the trinucleotide repeats (for review, see Rajender et al., 2007), it has been found that both ARQ and ARG have transcription activation modulating effect (Chamberlain et al., 1994, Lundin et al., 2007).
Monoamine oxidase is an enzyme located throughout the brain in the outer membrane of mitochondria, responsible for monoamine degradation. Monoamine oxidase A degrades mostly serotonin, norepinephrine and dopamine, while monoamine oxidase B has higher affinity toward phenylethylamine, benzylamine, and deprenyl (for review see Shih et al., 1999). The genes encoding these enzymes are located on the X-chromosome (MAOA and MAOB, respectively). Mutations in MAOA have been associated with aggressiveness and borderline mental retardation (Brunner et al., 1993; for review see Fan et al., 2010), while MAOB has been implied in Parkinson's disease (Hotamisligil et al., 1994, Liu et al., 2014). In humans, both genes have a polymorphic dinucleotide repeat motif in intron 2 (MAin2 and MBin2, respectively). MAin2 has been linked to bipolar disorder (Furlong et al., 1999, Kawada et al., 1995, Lin et al., 2000) and to attention-deficit hyperactivity disorder (Jiang et al., 2001), while MBin2 has been related to bipolar disorder (Lin et al., 2000) and to Parkinson's disease (Chan et al., 2003, Mellick et al., 2000). Even though the associations can be questioned given the intronic location of these loci (for example see Muramatsu et al., 1997), there is a strong possibility that MAin2 and MBin2 have biological functions that remain to be confirmed.
Behavioral trait- and disorder-related candidate genes have also been examined in animals, notedly in the phylogenetically closest animals to humans, the non-human primates (Anestis et al., 2014, Hopkins et al., 2012, Inoue-Murayama, 2009). The abovementioned four loci (ARQ, ARG, MAin2 and MBin2) have also been studied in non-human primates, including chimpanzees (Hong et al., 2006, Hong et al., 2008). However, as to the best of our knowledge, bonobos have never been studied for these four loci with the exception of 2 individuals for loci ARQ and ARG (Hong et al., 2006).
The divergence between bonobos and chimpanzees, the closest living relatives of humans, has been estimated to be approximately 1 million years ago (Hey, 2010). Bonobos and chimpanzees share several traits, such as living in fission–fusion social system and residing in a male philopatric society (Goodall, 1983, Kano, 1982), but the two species also differ in important ways that might be related to their aggressive tendencies. In contrast to chimpanzees, bonobo communities have lower levels of both intra- and inter-group aggression, while female bonobos have high social status, prolonged estrus periods and maintain strong social bonds with each other, all characteristics lacking in chimpanzees (Furuichi, 1997, Furuichi, 2011, Kano, 1992). Recently Hare et al. (2012) have proposed the self-domestication hypothesis, which speculates that the psychological, behavioral and morphological differences between bonobos and chimpanzees can be the consequences of selection against aggression, which is a typical characteristic of domestication.
Because AR, MAOA and MAOB influence aggressiveness along with other traits, they are among the ideal candidate genes to study the reasons for behavioral differences in chimpanzees and bonobos, which in turn can help us to understand the functions and evolution of these genes. As a first step toward this approach, we analyzed the loci ARQ, ARG, MAin2 and MBin2 in bonobos for comparison with those of chimpanzees and humans.
Materials and methods
Sample collection
Fecal sample collection took place in Wamba, Luo Scientific Reserve, Democratic Republic of the Congo between November 2012 and November 2013. The research camp is located at 0°11′08″ N, 22°37′58″ E. Details of the field site have been described (Hashimoto et al., 2008, Kano, 1992). Fecal samples were collected from 22 females and 10 males from 2 communities of wild bonobos (Pan paniscus), 13 females and 7 males from P group, and 9 females and 3 males from E1 group (for further information see Tokuyama et al., 2012 for P group, and Furuichi et al., 2012, Ryu et al., in press for E1 group). All the individuals in the analyzed communities were individually recognized. In P group, 4 females and 2 males were infants of 5 females, while in E1 group, a male was an infant of a female among the samples. The rest of the individuals were believed to be unrelated. The sampling procedure has been described by Kawamoto et al. (2013).
Genotyping
DNA was extracted by using a QIAamp DNA Stool Mini Kit (Qiagen, California, USA) according to the manufacturer's instructions. ARQ and ARG were amplified by applying the following primer sets: ARhf (5′-TCCAGAATCTGTTCCAGAGCGTGC-3′) and ARhr (5′-GCTGTGAGGGTTGCTGTTCCTCAT-3′) for ARQ, and ARGF (5′-CAGTGCCGCTATGGGGACCTGGCGA-3′) and ARGR (5′-GGACTGGGATAGGGCACTCTGCTCACC-3′) for ARG (Hong et al., 2006). The primer sets for monoamine oxidase were MAin2F (5′-TAGCTCCAGGAAGGAAATGTT-3′) and MAin2R (5′-CACCCAGGGAAAAGTAGGTTA-3′) for MAin2, and MBin2F (5′-GATTGGAAAGATTGTGTTGGTG-3′) and MBin2R (5′-CCAGTTTGCCTTCCTTTCAA-3′) for MBin2 (Hong et al., 2008). A 10 μl PCR reaction contained 2 μl DNA with a concentration of > 150 pg/μl (Step 3 in Table S1 in Kawamoto et al., 2013), 0.5 μM forward primer, 0.5 μM reverse primer, 0.5 U LA Taq polymerase, 400 μM of each of the dNTP and GC buffer I (TaKaRa, Shiga, Japan). Following the initial incubation at 95 °C for 2 min, PCR amplification was performed for 35 cycles of 95 °C for 30 s, 60 °C (ARQ) or 65 °C (ARG) for 1 min and 75 °C for 2 min for AR loci, while 35 cycles (MAin2) or 40 cycles (MBin2) of 95 °C for 30 s, 50 °C for 1 min and 75 °C for 2 min for MAOA and MAOB loci. For all loci PCR amplification was followed by a final extension at 74 °C for 10 min.
For genotyping we applied the ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, California, USA) according to the manufacturer's instructions by using labeled primers. We sequenced the major alleles in the ABI 3130xl Genetic Analyzer. Sequencing was repeated at least once in the case of each sequenced allele. We named the alleles by the number of repeats counted from nucleotide sequences or estimated from allele lengths. For androgen receptor, a registered human sequence (access no. NM_000044) and a registered chimpanzee sequence (access no. NM_001009012), for MAOA, a registered human sequence (access no. AB302097) and two registered chimpanzee sequences (access nos. AB302099 and AB302100AB302099AB302100), and for MAOB, two registered human sequences (access nos. X63276 and AB302115X63276AB302115), and two registered chimpanzee sequences (access nos. AB302116 and AB2117) were obtained from the GenBank database. Repeats were counted by the longest uninterrupted trinucleotide (androgen receptor) or dinucleotide repeats (monoamine oxidase). We used CLUSTAL W for sequence alignment (Thompson et al., 1994).
In order to compare the bonobo allele frequencies with those of chimpanzees and humans, we used the published allele frequency; in the case of androgen receptor 33 females and 19 males of humans (Comings et al., 1999), while 32 females and 25 males of chimpanzees (Hong et al., 2006), and in the case of MAOA and MAOB, 25 females and 30 males of humans, while 24 females and 18 males of chimpanzees (Hong et al., 2008).
To the best of our knowledge, none of the genes analyzed in this study have been analyzed in bonobos before. Hence we registered the nucleotide sequences in DDBJ/EMBL/GenBank nucleotide sequence database with the accession numbers AB970511–AB970525AB970511AB970512AB970513AB970514AB970515AB970516AB970517AB970518AB970519AB970520AB970521AB970522AB970523AB970524AB970525.
Statistical analysis
The statistical analysis was conducted using R software (version 2.15.2). The allele frequency was calculated as allele numbers/total number of chromosomes, that is 1 for males, 2 for females. To compare allele frequencies of the P and E1 communities, we used the chi-square test. Since we did not find any significant differences between the communities for any of the regions (p > 0.24), for the further analysis we handled the samples as belonging to one single population. We used two indices, Shannon's equitability (EH) and expected heterozygosity (He, Nei and Roychoudhury, 1974), to examine the diversity among humans, chimpanzees and bonobos on the loci ARQ, ARG, MAin2 and MBin2.
Results
The frequencies, Shannon's equitability and expected heterozygosity of the ARQ, ARG, MAin2 and MBin2 alleles in bonobos, along with those of humans and chimpanzees obtained from published data (Comings et al., 1999, Hong et al., 2006, Hong et al., 2008) are presented in Table 1, Table 2, Table 3, Table 4, respectively.
Table 1.
Allele frequencies of androgen receptor CAG repeats in human, chimpanzee and bonobo.
| ARQ | Human |
Chimpanzee |
Bonobo |
|---|---|---|---|
| N = 85 | N = 89 | N = 54 | |
| CAG11 | 0.037 | ||
| CAG12 | 0.019 | ||
| CAG14 | 0.023 | 0.045 | |
| CAG15 | 0.012 | 0.011 | 0.278 |
| CAG16 | 0.296 | ||
| CAG17 | 0.056 | 0.130 | |
| CAG18 | 0.326 | 0.241 | |
| CAG19 | 0.059 | 0.079 | |
| CAG20 | 0.109 | 0.011 | |
| CAG21 | 0.137a | 0.146a | |
| CAG22 | 0.117 | 0.112 | |
| CAG23 | 0.125 | 0.124 | |
| CAG24 | 0.098 | 0.045 | |
| CAG25 | 0.070 | 0.034 | |
| CAG26 | 0.102 | 0.011 | |
| CAG27 | 0.059 | ||
| CAG28 | 0.066 | ||
| CAG29 | 0.023 | ||
| He | 0.903 | 0.830 | 0.762 |
| EH | 1 | 0.852 | 0.634 |
Sequences of alleles with underlined frequencies were determined.
Human and chimpanzee allele frequencies were obtained from published data (human from Comings et al., 1999, chimpanzee from Hong et al., 2006).
He: the expected heterozygosity.
EH: the Shannon's equitability.
N: the number of X chromosomes.
GenBank accession numbers: human CAG21 NM_000044, chimpanzee CAG21 NM_001009012, bonobo CAG15–CAG18 AB970511–AB970514AB970511AB970512AB970513AB970514.
Table 2.
Allele frequencies of androgen receptor GGN repeats in human, chimpanzee and bonobo.
| ARG | Human |
Chimpanzee |
Bonobo |
|---|---|---|---|
| N = 85 | N = 89 | N = 54 | |
| GGN14 | 0.011 | ||
| GGN16 | 0.013 | ||
| GGN17 | 0.551a | ||
| GGN18 | 0.022 | 0.870 | |
| GGN19 | 0.089 | 0.393 | 0.130 |
| GGN20 | 0.011 | ||
| GGN22 | 0.032 | 0.011 | |
| GGN23 | 0.446a | ||
| GGN24 | 0.382 | ||
| GGN25 | 0.013 | ||
| GGN26 | 0.013 | ||
| GGN27 | 0.013 | ||
| He | 0.646 | 0.541 | 0.234 |
| EH | 1 | 0.726 | 0.309 |
Sequences of alleles with underlined frequencies were determined.
Human and chimpanzee allele frequencies were obtained from published data (human from Comings et al., 1999, chimpanzee from Hong et al., 2006).
Refer to Table 1 for abbreviations He, EH and N.
GenBank accession numbers: human GGN23 NM_000044, chimpanzee GGN17 NM_001009012, bonobo GGN18 AB970515, GGN19 AB970516.
Table 3.
Allele frequencies of monoamine oxidase A intron 2 dinucleotide repeats in human, chimpanzee and bonobo.
| MAOA | Human N = 80 |
Chimpanzee N = 66 |
Bonobo N = 54 |
|||
|---|---|---|---|---|---|---|
| Sequence | fr | Sequence | fr | Sequence | fr | |
| CN14 | (CA)10CG(CA)3 | 0.020 | ||||
| CN15 | (CA)11CG(CA)3 | 0.740a | ||||
| CN16 | (CA)12CG(CA)3 | 0.230a | (CN)16 | 0.037 | ||
| CN17 | (CA)13CG(CA)3 | 0.241 | ||||
| CN18 | (CA)14CG(CA)3 | 0.020 | (CA)14CG(CA)3 | 0.148 | ||
| CN19 | (CA)13(CGCA)2(CA)2 | 0.010 | (CA)15CG(CA)3 | 0.370 | ||
| CN20 | (CA)14(CGCA)2(CA)2 | 0.280a | ||||
| CN21 | (CA)15(CGCA)2(CA)2 | 0.160 | ||||
| CN22 | (CA)16(CGCA)2(CA)2 | 0.080 | (CA)22 | 0.111 | ||
| CN23 | (CA)17(CGCA)2(CA)2 | 0.060 | (CN)23 | 0.019 | ||
| CN24 | (CA)18(CGCA)2(CA)2 | 0.310a | (CN)24 | 0.019 | ||
| CN25 | (CA)19(CGCA)2(CA)2 | 0.090 | (CN)25 | 0.056 | ||
| CN26 | (CA)20(CGCA)2(CA)2 | 0.010 | ||||
| He | 0.782 | 0.399 | 0.766 | |||
| EH | 1 | 0.424 | 0.985 |
Sequences of alleles with underlined frequencies were determined.
Human and chimpanzee allele frequencies were obtained from published data (Hong et al., 2008).
Refer to Table 1 for abbreviations He, EH and N.
fr: frequency.
Table 4.
Allele frequencies of monoamine oxidase B intron 2 dinucleotide repeats in human, chimpanzee and bonobo.
| MAOB | Human N = 80 |
Chimpanzee N = 66 |
Bonobo N = 54 |
|||
|---|---|---|---|---|---|---|
| Sequence | fr | Sequence | fr | Sequence | fr | |
| GN19 | (GT)17(GA)2 | 0.010 | ||||
| GN20 | (GT)18(GA)2 | 0.140 | (GT)13(GA)7 | 0.370 | ||
| GN21 | (GT)19(GA)2 | 0.040 | (GT)15(GA)6 | 0.093 | ||
| GN22 | (GT)20(GA)2 | 0.040 | (GT)13(GA)9 | 0.020 | (GT)11(GA)11 | 0.074 |
| GN23 | (GT)21(GA)2 | 0.030 | (GT)12(GA)11 | 0.241 | ||
| GN24 | (GT)22(GA)2 | 0.080a | (GT)15(GA)9 | 0.740a | (GT)13(GA)11 | 0.222 |
| GN25 | (GT)23(GA)2 | 0.080 | (GT)16(GA)9 | 0.230a | ||
| GN26 | (GT)24(GA)2 | 0.150 | (GT)17(GA)9 | 0.020 | ||
| GN27 | (GT)25(GA)2 | 0.400a | ||||
| GN28 | (GT)26(GA)2 | 0.040 | ||||
| GN29 | (GT)27(GA)2 | 0.010 | ||||
| He | 0.779 | 0.399 | 0.743 | |||
| EH | 1 | 0.375 | 0.762 |
Sequences of alleles with underlined frequencies were determined.
Human and chimpanzee allele frequencies were obtained from published data (Hong et al., 2008).
Refer to Table 1 for abbreviations He, EH and N.
fr: frequency.
ARQ
We found 6 lengths in ARQ in bonobos, containing 11, 12, 15, 16, 17 and 18 CAG repeats (Table 1, typical peak patterns of PCR products in Supplementary Fig. S1). Bonobos had the fewest number of alleles per locus among the three species, in spite of the high values of expected heterozygosity and the Shannon's equitability (He = 0.762, EH = 0.634) compared with the other examined loci regarding all the three species.
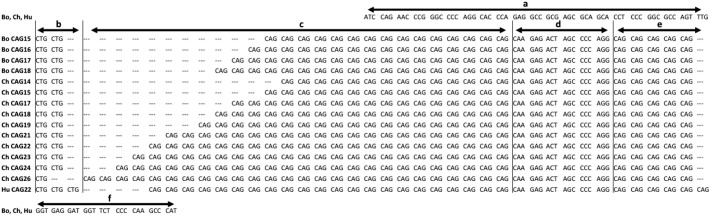
The nucleotide sequence alignments are shown in Fig. 1. A preceding sequence of 63 bp was uniform in bonobos, chimpanzees and humans, followed by a CTG repeat region that was (CTG)2 in bonobos, CTG or (CTG)2 in chimpanzees, while (CTG)3 in humans. The polymorphic CAG repeat region was followed by a monomorphic CAG repeat region: (CAG)5 in the Pan genus and (CAG)6 in humans. The sequence of 18 bp between the two CAG repeat regions was uniform in the three species. The following sequence of 27 bp was common in the three species.
Fig. 1.
Comparison of AR CAG repeats among bonobo, chimpanzee and human. Bo bonobo, Ch chimpanzee, Hu human. The number following CAG represents the repeat number of CAG trinucleotide. In the nucleotide sequence a: the preceding sequence that is common in bonobos, chimpanzees and humans, b: the preceding CTG repeats, c: the CAG repeats, d: a following sequence that is common in the three species, e indicates a second, non-polymorphic CAG repeat region, f: the following sequence that is common in the three species. GenBank accession numbers: human CAG21 NM_000044, chimpanzee CAG21 NM_001009012, bonobo CAG15–CAG18 AB970511–AB970514AB970511AB970512AB970513AB970514.
ARG
We detected 2 alleles in ARG in bonobos, containing 18 and 19 GGN repeats (Table 2, typical peak patterns in Supplementary Fig. S2). The (GGN)18 allele was common and the expected heterozygosity and the Shannon's equitability were small (He = 0.234, EH = 0.309) compared to those values of humans and chimpanzees.
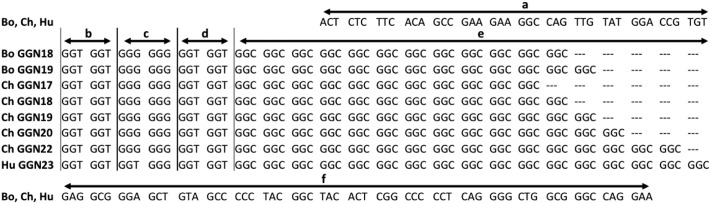
The nucleotide sequence alignments are shown in Fig. 2. A preceding sequence of 42 bp was identical in the three species. ARG had 4 motifs in bonobos (b–d in Fig. 3): (GGT)2, (GGG)2, (GGT)2 and (GGC)n from 5′ to 3′. This structure was identical to that of the chimpanzee, while in humans the second component of the motif was different: GGTGGG instead of (GGG)2. Repeat sequence was followed by a sequence of 63 bp that was uniform in the three species.
Fig. 2.
Comparison of AR GGN repeats among bonobo, chimpanzee and human. Bo bonobo, Ch chimpanzee, Hu human. The number following GGN represents the repeat number of GGN trinucleotide. In the nucleotide sequence a: the preceding sequence that is common in bonobos, chimpanzees and humans, b, c, d and e: the GGN repeat region, b: (GGT)2, c: (GGG)2 in the Pan genus and GGTGGG in human, d: (GGT)2, e: (GGC)n, f: the following sequence that is common in the three species. GenBank accession numbers: human GGN23 NM_000044, chimpanzee GGN17 NM_001009012, bonobo GGN18 AB970515, GGN19 AB970516.
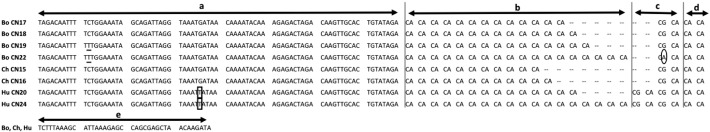
Fig. 3.
Comparison of MAOA CN repeats among bonobo, chimpanzee and human. Bo bonobo, Ch chimpanzee, Hu human. The number following CN represents the repeat number of CN dinucleotide. In the nucleotide sequence a: the preceding sequence that is identical in the three species with the exception of a C/T SNP in bonobos (underlined) and a monomorphic G → T single nucleotide change in humans (boxed), b: the CA repeat region, c: CGCA in the Pan genus with a G/A SNP in bonobo (circled), (CGCA)2 in human, d: (CA)2 in the three species, e: the following sequence that is common in the three species. GenBank accession numbers: human CN20 AB302097, CN24 AB302098, chimpanzee CN15 AB302099, CN16 AB302100, bonobo CN17–CN19, CN22 AB970517–AB970520AB970517AB970518AB970519AB970520.
MAin2
We uncovered 8 alleles in MAin2 in bonobos, containing 16, 17, 18, 19, 22, 23, 24 and 25 CN repeats (Table 3, typical peak patterns in Supplementary Fig. S3). The expected heterozygosity and the Shannon's equitability had high values (He = 0.766, EH = 0.985), exceeding the chimpanzee diversity values and almost equivalent to those of the human population.
The nucleotide sequence alignments are shown in Fig. 3. A preceding sequence of 78 bp was identical in the three species with the exception of a C/T SNP in bonobos and a monomorphic G → T single nucleotide change in humans (‘a’ in Fig. 3). MAin2 contained a polymorphic dinucleotide repeat (CA)n, followed by a single copy of (CGCA) motif in the Pan genus with a G/A SNP in bonobos (c in Fig. 3), while (CGCA)2 in humans, followed by a monomorphic (CA)2 motif in the three species. Repeat sequence was followed by a sequence of 38 bp that was equal in the three species.
MBin2
We discovered 5 alleles in MBin2 in bonobos, containing 20–24 GN repeats (Table 4, typical peak patterns in Supplementary Fig. S4). The expected heterozygosity and the Shannon's equitability values (He = 0.743, EH = 0.762) were greater than those of the chimpanzee, but remained below the human diversity values.
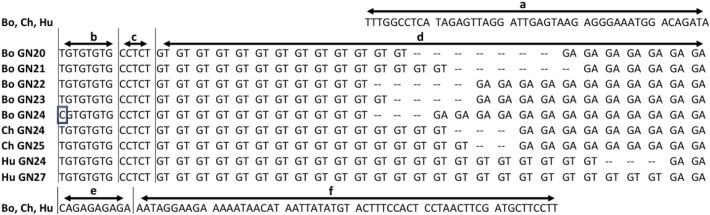
The nucleotide sequence alignments are shown in Fig. 4. A preceding sequence of 47 bp was common in the three species. The following (TG)4 motif with a C/T SNP in bonobos (b in Fig. 4) was followed by a motif of CCTCT in the three species. MBin2 had 2 motifs that showed different tendencies in the three species: (GT)n and (GA)n in the bonobo, (GT)n and (GA)9 in the chimpanzee, while (GT)n and (GA)2 in the human. The polymorphic GN repeat was followed by a (GA)4 motif separated by a CA dinucleotide. The following sequence of 60 bp was uniform in the three species.
Fig. 4.
Comparison of MAOB GN repeats among bonobo, chimpanzee and human. Bo bonobo, Ch chimpanzee, Hu human. The number following GN represents the repeat number of GN dinucleotide. In the nucleotide sequence a: the preceding sequence that is common in bonobos, chimpanzees and humans, b: (TG)4 with a T/C SNP in bonobos (boxed), c: common in the three species, d: the GN repeat region, e: CA(GA)4, f: the following sequence that is common in the three species. GenBank accession numbers: human GN24 X63276, GN27 AB302115, chimpanzee GN24 AB302116, GN25 AB2117, bonobo GN20–GN24 AB970521–AB970525AB970521AB970522AB970523AB970524AB970525.
Discussion
In wild bonobos, the four loci, ARQ, ARG, MAin2 and MBin2, were all polymorphic. Apart from ARG, where the study population had only 2 alleles, the loci were very diverse. We found the flanking regions of ARG and ARQ highly conservative as in chimpanzees and humans. The flanking regions of MAin2 and MBin2 were more diverse in bonobos than in chimpanzees or humans. Bonobos had 2 SNPs in the MAin2 locus and 1 SNP in the MBin2 locus, while no SNP was present in either humans or chimpanzees in these flanking regions. In the case of MBin2 the GN repeat region itself was more diverse than that of humans or chimpanzees. In the latter species, only the GT repeat region has been found to be polymorphic (Grimsby et al., 1992, Hong et al., 2008), while in the bonobos we found that the GA repeat region was also polymorphic. Loci MAin2 and MBin2 showed more allelic diversity in bonobos than in chimpanzees. Given that our bonobo population came from only two neighboring communities, while the chimpanzee population samples originated presumably from several communities (Hong et al., 2008), the result was surprising. Based on multiple intergenic autosomal regions, nucleotide diversity of bonobos has been found to be closest to western chimpanzees, who are characterized by the lowest nucleotide diversity among chimpanzee subspecies (Fischer et al., 2006, Fischer et al., 2011), which underlines our finding that bonobos showed higher diversity in the case of MAin2 and MBin2. Bonobos have lower level of aggression than chimpanzees (Furuichi, 2009, Hare et al., 2012). Because monoamine oxidase influences aggressiveness (Shih and Thompson, 1999), the differences between the polymorphisms of MAin2 and MBin2 in bonobos and chimpanzees may be associated with the differences in aggression between the two species.
ARQ and ARG have also been found to be associated with aggressiveness in humans (Vermeersch et al., 2010). We found that locus ARQ in bonobos had generally shorter alleles than in humans or chimpanzees. Such a finding could contradict other reports that more aggressive humans tended to have shorter CAG repeats (Jönsson et al., 2001). This unexpected result remains to be explained. Studies are needed focusing on the functions of the alleles in chimpanzees, bonobos and humans in order to understand the evolution of AR. Bonobos and chimpanzees are particularly adequate species to compare regarding AR polymorphisms, since it has been found that these two species were different in testosterone level change throughout ontogeny (Wobber et al., 2013).
The weak points of this study are that our bonobo samples originated from two neighboring communities in the wild, and 13 individuals were mother–offspring pairs among the 32, while the chimpanzee and human samples that we compared our results with originated from several populations. Even though this raises the significance of the higher diversity that we found in two of the four analyzed loci in bonobos, we have to consider our comparison with great care. The genetic diversity of bonobos shows extensive geographic variation (Kawamoto et al., 2013). Future studies investigating these loci in different bonobo populations are therefore recommended. Individual-based comparison is also necessary to elucidate the function of the candidate loci. For example Hong et al. (2011) have examined a polymorphism of tryptophan hydroxylase 2 gene in chimpanzees, and they reported that neuroticism was associated with it, as in humans.
In this study we found that bonobos were polymorphic on loci ARQ, ARG, MAin2 and MBin2, and that the diversity of the latter two loci was higher than in chimpanzees. Bonobos and chimpanzees differ in their level of aggressiveness, and aggressiveness could be associated with the genes that we analyzed, as in humans. Therefore, to understand the evolution of these loci and eventually AR, MAOA and MAOB in humans and non-human primates, it would be useful to conduct future studies on the association of aggressiveness and other personality traits with the polymorphisms found in this study in bonobos and to compare the results with those of chimpanzees and humans.
Acknowledgments
We are grateful for the members of the Primate Research Institute and Wildlife Research Institute of Kyoto University. We would like to express special thanks to the trackers and workers of the Wamba field site and Dr. Tetsuya Sakamaki for their help in the field. Our sincere thanks also go to Dr. Fred Bercovitch for his invaluable comments on the manuscript. We thank the three anonymous reviewers for their valuable suggestions. This work was supported by the Cooperation Research Program of Wildlife Research Center, Kyoto University (grant numbers 2013-shisetsu-4 and 2014-shisetsu-3 to Dr. Miho Inoue-Murayama), by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) with a Grant-in-aid for Science Research (grant numbers 21310150, 25290082, and 25118005 to Dr. Miho Inoue-Murayama), by the ITP-HOPE project (ITP-25-004 to Cintia Garai) and by the AS-HOPE project (AS-24-034 to Cintia Garai). We sincerely thank the Research Center for Ecology and Forestry, and Ministry of Scientific Research, DRC for helping our field research. We are grateful for the Environment Research and Technology Development Fund (D-1007 to Dr. Takeshi Furuichi), the Japan Society for the Promotion of Science (JSPS) Asia–Africa Science Platform Program (2009–2011, 2012–2014 to Dr. Takeshi Furuichi), and the JSPS Grants-in-aid for Scientific Research (22255007 to Dr. Takeshi Furuichi) for the support in the maintenance of the Wamba study site and the employment of research assistants.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.mgene.2014.10.005.
Appendix A. Supplementary data
Fig. S1 Peak patterns of locus ARQ in bonobos.
Fig. S2 Peak patterns of locus ARG in bonobos.
Fig. S3 Peak patterns of locus MAin2 in bonobos.
Fig. S4 Peak patterns of locus MBin2 in bonobos.
References
- Aluja A., García L.F., Blanch A., Fibla J. Association of androgen receptor gene, CAG and GGN repeat length polymorphism and impulsive-disinhibited personality traits in inmates: the role of short–long haplotype. Psychiatr. Genet. 2011;21:229–239. doi: 10.1097/YPG.0b013e328345465e. [DOI] [PubMed] [Google Scholar]
- Anestis S.F., Webster T.H., Kamilar J.M., Fontenot M.B., Watts D.P., Bradley B.J. AVPR1A variation in chimpanzees (Pan troglodytes): population differences and association with behavioral style. Int. J. Primatol. 2014;35:305–324. [Google Scholar]
- Brunner H.G., Nelen M., Breakefield X.O., Ropers H.H., van Oost B.A. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Chamberlain N.L., Driver E.D., Miesfeld R.L. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D.K., Mellick G.D., Hung W.T., Woo J. Genetic and environmental risk factors and their interactions for Parkinson's disease in a Chinese population. J. Clin. Neurosci. 2003;10:313–315. doi: 10.1016/s0967-5868(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Chang C.S., Kokontis J., Liao S.T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc. Natl. Acad. Sci. U. S. A. 1988;85:7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D.E., Chen C., Wu S., Muhleman D. Association of the androgen receptor gene (AR) with ADHD and conduct disorder. Neuroreport. 1999;10:1589–1592. doi: 10.1097/00001756-199905140-00036. [DOI] [PubMed] [Google Scholar]
- D'Souza U.M., Craig I.W. Functional polymorphisms in dopamine and serotonin pathway genes. Hum. Mutat. 2006;27:1–13. doi: 10.1002/humu.20278. [DOI] [PubMed] [Google Scholar]
- Ebstein R.P. The molecular genetic architecture of human personality: beyond self-report questionnaires. Mol. Psychiatry. 2006;11:427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Fan M., Liu B., Jiang T., Jiang X., Zhao H., Zhang J. Meta-analysis of the association between the monoamine oxidase-A gene and mood disorders. Psychiatr. Genet. 2010;20:1–7. doi: 10.1097/YPG.0b013e3283351112. [DOI] [PubMed] [Google Scholar]
- Fischer A., Pollack J., Thalmann O., Nickel B., Paabo S. Demographic history and genetic differentiation in apes. Curr. Biol. 2006;16:1133–1138. doi: 10.1016/j.cub.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Fischer A., Prufer K., Good J.M., Halbwax M., Wiebe V., Andre C., Atencia R., Mugisha L., Ptak S.E., Paabo S. Bonobos fall within the genomic variation of chimpanzees. PLoS ONE. 2011;6(6):e21605. doi: 10.1371/journal.pone.0021605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong R.A., Ho L., Rubinsztein J.S., Walsh C., Paykel E.S., Rubinsztein D.C. Analysis of the monoamine oxidase A (MAOA) gene in bipolar affective disorder by association studies, meta-analyses, and sequencing of the promoter. Am. J. Med. Genet. 1999;88:398–406. [PubMed] [Google Scholar]
- Furuichi T. Agonistic interactions and matrifocal dominance rank of wild bonobos (Pan paniscus) at Wamba. Int. J. Primatol. 1997;18:855–875. [Google Scholar]
- Furuichi T. Factors underlying party size differences between chimpanzees and bonobos: a review and hypotheses for future study. Primates. 2009;50:197–209. doi: 10.1007/s10329-009-0141-6. [DOI] [PubMed] [Google Scholar]
- Furuichi T. Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. 2011;20:131–142. doi: 10.1002/evan.20308. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Idani G., Ihobe H., Hashimoto C., Tashiro Y., Sakamaki T., Mulavwa M.N., Yangozene K., Kuroda S. Long-term studies on wild bonobos at Wamba, Luo Scientific Reserve, D. R. Congo: towards the understanding of female life history in a male-philopatric species. In: Kappeler P.M., Watts D.P., editors. Long-Term Field Studies of Primates. Springer; Heidelberg: 2012. pp. 413–433. [Google Scholar]
- Goodall J. Population dynamics during a 15-year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Z. Tierpsychol. 1983;61:1–60. [Google Scholar]
- Grimsby J., Chen K., Devor E.J., Cloninger C.R., Shih J.C. Dinucleotide repeat (TG)23 polymorphism in the MAOB gene. Nucleic Acids Res. 1992;20:924. doi: 10.1093/nar/20.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B., Wobber V., Wrangham R. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Anim. Behav. 2012;83:573–585. [Google Scholar]
- Hashimoto C., Tashiro Y., Hibino E., Mulavwa M., Yangozene K., Furuichi T., Idani G., Takenaka O. Longitudinal structure of a unit-group of bonobos: male philopatry and possible fusion of unit-groups. In: Furuichi T., Thompson J., editors. The Bonobos: Behavior, Ecology, and Conservation. Springer; New York: 2008. pp. 107–119. [Google Scholar]
- Hey J. The divergence of chimpanzee species and subspecies as revealed in multipopulation isolation-with-migration analyses. Mol. Biol. Evol. 2010;27:921–933. doi: 10.1093/molbev/msp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.W., Hibino E., Takenaka O., Hayasaka I., Murayama Y., Ito S., Inoue-Murayama M. Comparison of androgen receptor CAG and GGN repeat length polymorphism in humans and apes. Primates. 2006;47:248–254. doi: 10.1007/s10329-005-0174-4. [DOI] [PubMed] [Google Scholar]
- Hong K.W., Hayasaka I., Murayama Y., Ito S., Inoue-Murayama M. Comparative analysis of monoamine oxidase intronic polymorphisms in primates. Gene. 2008;418:9–14. doi: 10.1016/j.gene.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Hong K.W., Weiss A., Morimura N., Udono T., Hayasaka I., Humle T., Murayama Y., Ito S., Inoue-Murayama M. Polymorphism of the tryptophan hydroxylase 2 (TPH2) gene is associated with chimpanzee neuroticism. PLoS ONE. 2011;6(7):e22144. doi: 10.1371/journal.pone.0022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W.D., Donaldson Z.R., Young L.J. A polymorphic indel containing the RS3 microsatellite in the 5′ flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes Brain Behav. 2012;11:552–558. doi: 10.1111/j.1601-183X.2012.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S., Girmen A.S., Fink J.S., Tivol E., Shalish C., Trofatter J., Baenziger J., Diamond S., Markham C., Sullivan J., Growdon J., Breakefield X.O. Hereditary variations in monoamine oxidase as a risk factor for Parkinson's disease. Mov. Disord. 1994;9:305–310. doi: 10.1002/mds.870090304. [DOI] [PubMed] [Google Scholar]
- Inoue-Murayama M. Genetic polymorphism as a background of animal behavior. Anim. Sci. J. 2009;80:113–120. doi: 10.1111/j.1740-0929.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- Jiang S., Xin R., Lin S., Qian Y., Tang G., Wang D., Wu X. Linkage studies between attention-deficit hyperactivity disorder and the monoamine oxidase genes. Am. J. Med. Genet. 2001;105:783–788. doi: 10.1002/ajmg.10098. [DOI] [PubMed] [Google Scholar]
- Jönsson E.G., von Gertten C., Gustavsson J.P., Yuan Q.P., Lindblad-Toh K., Forslund K., Rylander G., Mattila-Evenden M., Asberg M., Schalling M. Androgen receptor trinucleotide repeat polymorphism and personality traits. Psychiatr. Genet. 2001;11:19–23. doi: 10.1097/00041444-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Kano T. The social group of pygmy chimpanzees (Pan paniscus) of Wamba. Primates. 1982;23:171–188. [Google Scholar]
- Kano T. Stanford Univ. Press; Stanford: 1992. The Last Ape: Pygmy Chimpanzee Behavior and Ecology. [Google Scholar]
- Kawada Y., Hattori M., Dai X.Y., Nanko S. Possible association between monoamine oxidase A gene and bipolar affective disorder. Am. J. Hum. Genet. 1995;56:335–336. [PMC free article] [PubMed] [Google Scholar]
- Kawamoto Y., Takemoto H., Higuchi S., Sakamaki T., Hart J.A., Hart T.B., Tokuyama N., Reinartz G.E., Guislain P., Dupain J., Cobden A.K., Mulavwa M.N., Yangozene K., Darroze S., Devos C., Furuichi T. Genetic structure of wild bonobo populations: diversity of mitochondrial DNA and geographical distribution. PLoS ONE. 2013;8(3):e59660. doi: 10.1371/journal.pone.0059660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Jiang S., Wu X., Qian Y., Wang D., Tang G., Gu N. Association analysis between mood disorder and monoamine oxidase gene. Am. J. Med. Genet. 2000;96:12–14. doi: 10.1002/(sici)1096-8628(20000207)96:1<12::aid-ajmg4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang Z., Zhang B. The relationship between monoamine oxidase B (MAOB) A644G polymorphism and Parkinson disease risk: a meta-analysis. Ann. Saudi Med. 2014;34:12–17. doi: 10.5144/0256-4947.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin K.B., Giwercman A., Dizeyi N., Giwercman Y.L. Functional in vitro characterisation of the androgen receptor GGN polymorphism. Mol. Cell. Endocrinol. 2007;264:184–187. doi: 10.1016/j.mce.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Mellick G.D., Buchanan D.D., Silburn P.A., Chan D.K., Le Couteur D.G., Law L.K., Woo J., Pang C.P. The monoamine oxidase B gene GT repeat polymorphism and Parkinson's disease in a Chinese population. J. Neurol. 2000;247:52–55. doi: 10.1007/s004150050010. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Matsushita S., Kanba S., Higuchi S., Manki H., Suzuki E., Asai M. Monoamine oxidase genes polymorphisms and mood disorder. Am. J. Med. Genet. 1997;74:494–496. [PubMed] [Google Scholar]
- Nei M., Roychoudhury A.K. Sampling variances of heterozygosity and genetic distance. Genetics. 1974;76(2):379–390. doi: 10.1093/genetics/76.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajender S., Singh L., Thangaraj K. Phenotypic heterogeneity of mutations in androgen receptor gene. Asian J. Androl. 2007;9:147–179. doi: 10.1111/j.1745-7262.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- Ryu H., Hill D.A., Furuichi T. Prolonged maximal sexual swelling in wild bonobos facilitates affiliative interactions between females. Behaviour. 2014 (in press) [Google Scholar]
- Shih J.C., Thompson R.F. Monoamine oxidase in neuropsychiatry and behavior. Am. J. Hum. Genet. 1999;65:593–598. doi: 10.1086/302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J.C., Chen K., Ridd M.J. Monoamine oxidase: from genes to behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama N., Emikey B., Bafike B., Isolumbo B., Iyokango B., Mulavwa M.N., Furuichi T. Bonobos apparently search for a lost member injured by a snare. Primates. 2012;53:215–219. doi: 10.1007/s10329-012-0298-2. [DOI] [PubMed] [Google Scholar]
- Vermeersch H., T'Sjoen G., Kaufman J.M., Vincke J., Van Houtte M. Testosterone, androgen receptor gene CAG repeat length, mood and behaviour in adolescent males. Eur. J. Endocrinol. 2010;163:319–328. doi: 10.1530/EJE-10-0090. [DOI] [PubMed] [Google Scholar]
- Westberg L., Henningsson S., Landén M., Annerbrink K., Melke J., Nilsson S., Rosmond R., Holm G., Anckarsäter H., Eriksson E. Influence of androgen receptor repeat polymorphisms on personality traits in men. J. Psychiatry Neurosci. 2009;3:205–213. [PMC free article] [PubMed] [Google Scholar]
- Wobber V., Hare B., Lipson S., Wrangham R., Ellison P. Different ontogenetic patterns of testosterone production reflect divergent male reproductive strategies in chimpanzees and bonobos. Physiol. Behav. 2013;116–117:44–53. doi: 10.1016/j.physbeh.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Zhai X.L., Qu X.W., Guo L., Ha Q.H. Correlation study between the polymorphism of repetitive sequence in gene CAG of androgen receptor and the occurrence and progression of prostate cancer. Asian Pac. J. Trop. Med. 2014;4:301–304. doi: 10.1016/S1995-7645(14)60043-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Peak patterns of locus ARQ in bonobos.
Fig. S2 Peak patterns of locus ARG in bonobos.
Fig. S3 Peak patterns of locus MAin2 in bonobos.
Fig. S4 Peak patterns of locus MBin2 in bonobos.