Figure 6. Repression of C-terminal phosphoforms of β-CATENIN and of CYCLIN D1 by Ivermectin and Selamectin and their rescue by phophatase inhibition.
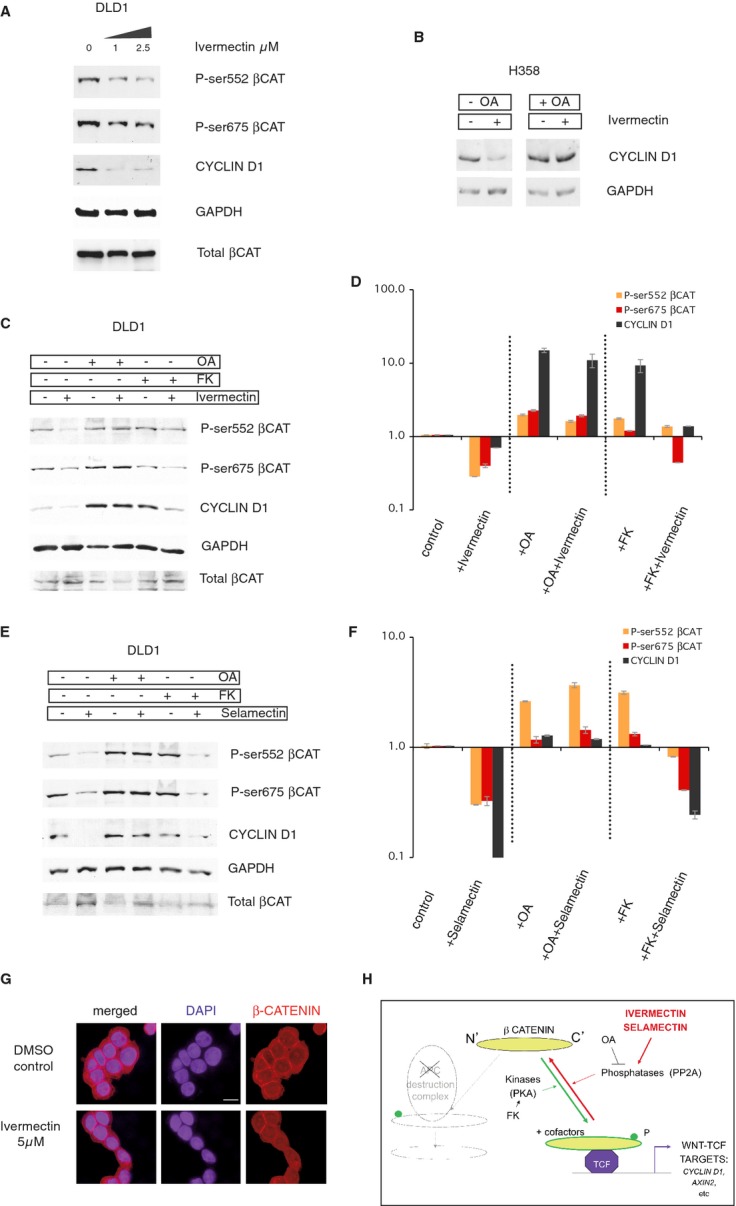
A Western blot of DLD1 human colon cancer cell extracts showing the concentration-dependent loss of C-terminal phosphoforms of β-CATENIN (P-Ser552 and P-Ser675), and of the levels of the TCF target CYCLIN D1, by Ivermectin at 1 and 2.5 μM. GAPDH and total β-CATENIN levels are shown as controls.
B The repressive effect of Ivermectin (5 μM) on CYCLIN D1 levels, used here as a signature for final TCF output, is also observed in lung cancer H358 cells, and this is rescued by okadaic acid (15 nM) treatment.
C–F Western blots (C, E) and their quantification (D, F) showing the rescue of the inhibitory effect of Ivermectin (5 μM) (C, D) and Selamectin (0.5 μM) (E, F) by OA (15 nM) but not by FK (10 μM) treatment. GAPDH levels are shown as loading controls in all panels. All treatments were for 12 h to highlight early responses.
G Subcellular localization of total β-CATENIN protein in control CC14 cells (top) and in those treated with Ivermectin (5 μM) for 6 h. The panels show confocal microscopy images for β-CATENIN (right), DAPI highlighting the nuclei (center) and merged images (left). Scale bar = 15 μm for all panels in (G).
H Diagram of the transition of C-terminally unphosphorylated β-CATENIN to its phosphorylated version, which with TCF and co-factors activate the transcription of target genes including notably that of CYCLIN D1. Phosphorylation is promoted by protein kinases such as Protein Kinase A (PKA), which is activated by Forskolin (FK), and inhibited by phosphatases such as PP2A that is inhibited by okadaic acid (OA). The action of Ivermectin and Selamectin is thus suggested to require active phosphatases.
Data information: Error bars = s.e.m.
