INTRODUCTION
Prevention of mother to child HIV transmission (MTCT) has been a resounding public health success story. In the past 20 years, the rate of MTCT in the US has been reduced from 26% to under 1% using potent combination antiretroviral (cARV) regimens [1-4]. As the use of cARV regimens in pregnancy has increased, concerns have been raised regarding the potential risk of in utero cARV exposure on long-term outcomes among HIV-exposed but uninfected (HEU) children, including mitochondrial toxicity which has also been associated with cardiomyopathy in HIV-unexposed children [5-12].
Among HIV-infected children, cardiomyopathy was common prior to widespread use of cARV and was associated with high mortality [13]. However, a study of both HEU and HIV-infected children in the National Heart, Lung, and Blood Institute (NHLBI) Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study (P2C2) born between 1990-1994 found no association between perinatal exposure to zidovudine (ZDV) monotherapy and abnormalities of left ventricular (LV) structure or function [14]. In another report, compared to the subset of ARV-unexposed HEU children in the P2C2 study, 136 children (96% exposed to cARV in utero) born between 2003-2006 in the NHLBI-funded Cardiovascular Status of Highly Active Antiretroviral Therapy (HAART) in HIV-Exposed Infants and Children cohort study (CHAART I) had significantly lower LV mass, septal wall thickness, and LV diameter, and higher LV contractility at age 2 years [15].
Since these prior studies were limited by their small sample size and/or they were conducted during an earlier era when there were fewer antiretroviral (ARV) options compared to the contemporary era which has more robust combination ARV combination therapy options, we evaluated the relationship between perinatal ARV drug exposure and echocardiographic measurements from the Pediatric HIV/AIDS Cohort Study (PHACS) Surveillance Monitoring for ART Toxicities (SMARTT) study.
METHODS
Study Population
The PHACS SMARTT study is a prospective cohort study designed to identify adverse effects of in utero ARV exposures on HEU children at 22 US pediatric HIV clinical centers. A reference cohort of children born to HIV-uninfected mothers was enrolled from the same study sites. The SMARTT protocol was reviewed and approved by the institutional review boards (IRB) at all participating clinical sites and the PHACS Data and Operations Center at the Harvard School of Public Health. Written informed consent was obtained from the children’s parent or legal guardian, and minor assent per site IRB requirements.
Maternal ARV regimen and substance use (illicit drugs, alcohol, and tobacco) by trimester were collected at study entry or from participation in previous studies including the PACTG 219C study and the Women and Infant Transmission Study [4]. After enrollment, annual SMARTT child study visits included physical exams and collection of new diagnoses and caregiver information.
Study Echocardiograms
To achieve appropriate statistical power in addressing our scientific question, we determined that we needed to perform a single echocardiogram on 400 HEU children in the SMARTT cohort at the first study visit between age 3 and 5 years. All site sonographers received specific protocol training. Using 2-dimensional and M-mode echocardiography, measures of cardiac function (LV ejection fraction, fractional shortening, and stress velocity index) and structural parameters (LV end diastolic (ED) short axis dimension, posterior wall thickness, septal thickness, LV mass, and thickness-to-dimension ratio, and end systolic (ES) wall stress) were measured. All digitized echocardiograms were assessed for image quality and were centrally measured at the PHACS echocardiographic core laboratory at Boston Children’s Hospital (S. Colan). The echocardiographic core laboratory was blinded to patient, prior therapy, and echocardiographic measurements performed at the study centers. The HIV-unexposed reference cohort underwent a single echocardiogram at ages 3-5 years. The only exclusion criterion for this analysis was presence of a significant congenital heart defect (2 [2%] children excluded from the HIV-unexposed reference cohort and 11 [2.7%] from the HEU cohort). All echocardiographic parameters were expressed as Z-scores to adjust for variation in age and body surface area in the cohorts. The Z-scores for both SMARTT cohorts were calculated using normative data from a healthy pediatric reference cohort from the echocardiography laboratory at Boston Children’s Hospital (BCH). The designation of “healthy” was determined by a detailed clinical evaluation and medical record review 1 year after the echocardiographic evaluation. The BCH reference cohort was more likely to be non-Hispanic white and may not have been from the same socioeconomic strata as the SMARTT cohorts [16].
Child and Maternal Factors and Maternal Antiretroviral Use
Child demographic factors, body mass index, birth characteristics at the time of study echocardiogram as well as maternal characteristics including substance use during pregnancy, age at delivery, and HIV viral load and CD4 count prior to delivery were considered as potential confounders (Table 1). cARV was defined as use of at least 3 ARV drugs. We also evaluated the subset of cARV regimens defined as HAART (≥ 3 ARV drugs from ≥ 2 drug classes) but results were similar to those of cARV and are not presented here. Intrauterine exposures to individual ARV drugs, ARV drug class, and cARV were examined overall and by first trimester exposure as this is the most critical period of cardiac development. ARV drug classes included nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs).
Table 1.
Child and maternal characteristics for 3 to 5 year old children with an echocardiogram (PHACS SMARTT Study, United States, 2006-2013)
| Characteristica | SMARTT Cohort
|
P Value b | |
|---|---|---|---|
| HIV-exposed uninfected (HEU) cohort (n = 417) | HIV-unexposed reference cohort (n = 98) | ||
| Age at echocardiogram (years), median (min, max) | 4.0 (2.8, 7.4) | 4.8 (2.8, 5.9) | 0.53 |
| Female | 207 (50%) | 52 (53%) | 0.58 |
| Race | 0.16 | ||
| Black/African-American | 257 (62%) | 69 (70%) | |
| White | 123 (30%) | 25 (26%) | |
| Other/not reported | 37 (9%) | 4 (4%) | |
| Hispanic ethnicity | 161 (39%) | 22 (22%) | 0.002 |
| Child’s BMI at echocardiogram, mean (SD) | 16.7 (2.3) | 15.7 (2.4) | <0.001 |
| Child’s birth characteristics | |||
| Birth weight <2.5kg | 84 (20%) | 15 (16%) | 0.47 |
| Gestational age <37 weeks | 82 (20%) | 11 (12%) | 0.10 |
| Small for gestational age (<10th percentile weight for gestational age) | 44 (11%) | 9 (10%) | 1.00 |
| Mother’s age at delivery (years) | |||
| Mean (SD) | 28.2 (6.0) | 26.2 (6.4) | 0.002 |
| <25 years at delivery | 124 (30%) | 50 (55%) | <0.001 |
| Mother’s VL >1000 cpm prior to delivery | 55 (14%) | --- | --- |
| Mother’s CD4 count <200 cells/mm3 prior to delivery | 32 (8%) | --- | --- |
| Maternal substance use during pregnancy | |||
| Tobacco | 76 (20%) | 10 (11%) | 0.067 |
| Alcohol | 26 (7%) | 4 (4%) | 0.63 |
| Illicit drugs | 37 (10%) | 4 (4%) | 0.15 |
BMI = body mass index, SD = standard deviation, VL = viral load, cpm = copies per milliliter
Data on certain characteristics were unavailable for reference and HEU subjects, for ethnicity (n = 0 and 1), birth weight (n = 5 and 0), gestational age (n = 6 and 2), SGA (n = 12 and 17), maternal age (n = 7 and 0), substance use (n = 9 and 32), and for HEU, maternal VL (n = 22) and CD4 (n = 25).
P value by Fisher’s exact test for binary characteristics, Chi-Square test for categorical characteristics (race), and Wilcoxon rank-sum test for continuous characteristics
The SMARTT HEU cohort echocardiographic parameters were descriptively compared to results from HEU children enrolled in the prior P2C2 HIV study (1990-1994) which serves as a cARV-unexposed historical comparison group, and the CHAART I study (2003-2006) which serves as a comparably cARV-exposed group to the SMARTT cohort from an earlier ARV treatment era. In the P2C2 cohort, 34% were exposed to ZDV and the rest were unexposed to ARV. In the CHAART I cohort, 96% were exposed to cARV. Detailed methods of these studies have been previously published [17,18]. All P2C2 and CHAART I echocardiograms were also centrally re-measured at the PHACS echocardiography core laboratory. Access to the P2C2 and CHAART I databases was provided via inter-institutional data use agreements between the University of Miami, Clinical Trials & Survey Corporation (Owings Mills, MD) and the Harvard School of Public Health.
Statistical Analysis
The Z-scores of the echocardiographic parameters were summarized by HIV exposure status and compared between HEU and HIV-unexposed groups using a two-sample t test. Among those in the HEU cohort with detailed information on maternal ARV exposure, unadjusted linear regression models were first used to assess associations between these Z-scores and in utero ARV exposures. For each echocardiographic parameter, a core model of potential confounders (child demographic variables and maternal factors) was built using a backward selection procedure including variables with a P value < 0.20 in univariable models. Pregnancy outcomes (e.g., low birth weight, prematurity) were not considered for the selection procedure due to concerns that they may be on the causal pathway between in utero ARV exposure and echocardiographic outcomes. All covariates with P value < 0.15 were retained as part of the core model of potential confounders. Linear regression models were then fit to assess the association between in utero ARV exposures and echocardiographic Z-scores, adjusting for the core model covariates. In a sensitivity analysis, the models were restricted to children with cARV exposure as opposed to any ARV exposure. Finally, the Z-scores of selected echocardiographic parameters from the P2C2 and CHAART studies were summarized and displayed graphically, along with those from the SMARTT cohorts.
Since SMARTT is a drug safety study, we prioritized limiting Type II statistical errors (falsely missing a safety signal) rather than Type I errors (falsely rejecting the null hypothesis) and thus no adjustments for multiple testing were used [19-22]. Statistical analyses were conducted using SAS Version 9.2 (SAS Institute, Cary, NC) using data submitted as of April 2012, and two-sided P values ≤ 0.05 were considered statistically significant.
RESULTS
Between 2007 and 2012, 428 (74%) HEU and 100 (100%) HIV-unexposed had an echocardiogram performed, meeting the study’s target sample sizes for echocardiograms. After excluding subjects with a congenital cardiac malformation, child and maternal characteristics are compared between the remaining 417 SMARTT HEU and 98 HIV-unexposed children in Table 1. The two groups were generally similar, although the mothers in the HIV-unexposed reference cohort were younger at parturition and had a trend for less often reported tobacco use during pregnancy compared to mothers in the HEU cohort, and the children were leaner and less likely Hispanic and had a trend for lower rate of preterm delivery.
A comparison of select echocardiographic Z-scores between the SMARTT HEU and HIV-unexposed reference cohorts and the CHAART I and P2C2 HEU children is shown in Figure 1. The P2C2 HEU cohort had markedly lower LV function (LV shortening fraction and stress velocity index Z-scores) and a much greater septal thickness compared to the other three groups. There were no significant differences in mean Z-scores between the SMARTT HEU and HIV-unexposed cohorts for any echocardiographic parameter (Table 2 and Figure 1), whether with or without adjustment for characteristics which differed between the two cohorts.
Figure 1.
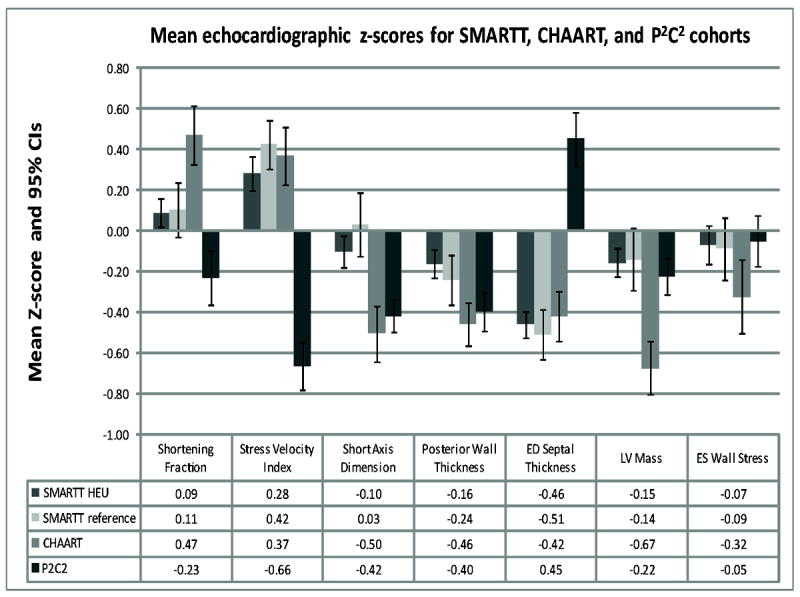
Echocardiographic Z-score comparisons between the SMARTT HIV-exposed uninfected (SMARTT HEU) and HIV-unexposed (SMARTT HIV-unexposed) cohorts and results from the previous NHLBI-funded CHAART and P2C2 studies. CI = confidence interval; ED = end diastolic; LV = left ventricular; ES = end systolic.
Table 2.
Comparison of mean echocardiographic parameter Z-scores between the SMARTT HIV-exposed uninfected cohort and the HIV-unexposed reference cohort
| Echocardiographic Z-score | SMARTT Cohort | Unadjusted Comparison | Adjusted Comparisona | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HIV-exposed uninfected (HEU) cohort (n = 417) Mean (SE), N | HIV-unexposed reference cohort (n = 98) Mean (SE), N | Mean Difference (95% CI) | P Value | Adjusted Mean Difference (95% CI) | P Value | |
| FUNCTIONAL MEASURES | ||||||
| LV ejection fraction | 0.20 (0.04), 398 | 0.29 (0.07), 92 | 0.09 (-0.07, 0.26) | 0.26 | 0.04 (-0.14, 0.21) | 0.70 |
| LV M-mode shortening fraction | 0.09 (0.04), 413 | 0.11 (0.08), 98 | 0.02 (-0.17, 0.20) | 0.86 | -0.06 (-0.26, 0.15) | 0.57 |
| LV stress velocity index | 0.28 (0.05), 370 | 0.42 (0.10), 91 | 0.14 (-0.07, 0.35) | 0.18 | 0.12 (-0.11, 0.35) | 0.30 |
| STRUCTURAL PARAMETERS | ||||||
| LV M-mode ED short axis dimension | -0.10 (0.05), 412 | 0.03 (0.09), 98 | 0.13 (-0.07, 0.34) | 0.20 | 0.07 (-0.15 0.29) | 0.52 |
| LV M-mode ED post wall thickness | -0.16 (0.04), 412 | -0.24 (0.08), 98 | -0.08 (-0.26, 0.10) | 0.39 | -0.05 (-0.25, 0.15) | 0.65 |
| M-mode ED septal thickness | -0.46 (0.04), 412 | -0.51 (0.08), 98 | -0.05 (-0.22, 0.13) | 0.59 | -0.06 (-0.25, 0.13) | 0.51 |
| LV M-mode mass | -0.15 (0.04), 412 | -0.14 (0.09), 98 | 0.02 (-0.18, 0.21) | 0.87 | -0.02 (-0.23, 0.19) | 0.83 |
| LV M-mode ES wall stress | -0.07 (0,05), 376 | -0.09 (0.11), 93 | -0.02 (-0.26, 0.22) | 0.89 | -0.02 (-0.29, 0.25) | 0.88 |
| LV M-mode thickness-to-dimension ratio | -0.52 (0.04), 412 | -0.64 (0.08), 98 | -0.12 (-0.30, 0.05) | 0.16 | -0.07 (-0.26, 0.12) | 0.46 |
LV = left ventricular; ED = end-diastolic; ES = end-systolic; SE=standard error; CI=confidence interval
Adjusted linear regression models include Hispanic ethnicity, child body mass index, younger maternal age (<25yrs) at delivery, and maternal tobacco use during pregnancy.
All further results are limited to the 411 SMARTT HEU participants with information on timing of maternal ARV exposure.
Left ventricular function measures
(Table 3) Among the SMARTT HEU participants, there were no statistically significant adjusted mean differences in Z-scores for three measures of LV function (fractional shortening, ejection fraction and stress-velocity index) when those exposed anytime during pregnancy to a specific ARV regimen or individual ARV drugs were compared to those unexposed to that ARV regimen or specific ARV drug. However, first trimester exposures to cARV, tenofovir, emtricitabine, or lopinavir/ritonavir were each associated with significantly lower mean LV stress velocity index Z-scores (a load-independent measure of LV contractility [23]) compared to those not exposed to these regimens or individual ARV drugs, with mean decreases of 0.22 to 0.40 standard deviations (SD).
Table 3.
Adjusted differences in functional echocardiographic parameter mean Z-scores for 411 HIV-exposed uninfected children in SMARTT exposed vs. unexposed in utero to specific antiretroviral regimens or drugs
| ARV Exposure | Percent exposed |
Left Ventricular Functional Echocardiographic Parametera
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ejection Fraction | Fractional Shortening | Stress Velocity Index | ||||||||
|
| ||||||||||
| Estimated mean difference |
(95% CI) | P Value | Estimated mean difference |
(95% CI) | P Value | Estimated mean difference |
(95% CI) | P Value | ||
|
Anytime during pregnancy
| ||||||||||
| cARV | 95% | 0.26 | (-0.09, 0.61) | 0.14 | 0.03 | (-0.40, 0.46) | 0.90 | -0.11 | (-0.60, 0.37) | 0.64 |
| NRTIs | ||||||||||
| Zidovudine | 82% | 0.06 | (-0.14, 0.26) | 0.55 | -0.00 | (-0.24, 0.23) | 0.98 | 0.23 | (-0.04, 0.49) | 0.10 |
| Lamivudine | 85% | 0.07 | (-0.14, 0.28) | 0.50 | 0.04 | (-0.21, 0.30) | 0.74 | 0.04 | (-0.25, 0.33) | 0.79 |
| Abacavir | 28% | 0.02 | (-0.14, 0.18) | 0.84 | 0.07 | (-0.13, 0.27) | 0.49 | 0.03 | (-0.19, 0.25) | 0.80 |
| Didanosine | 6% | 0.11 | (-0.22, 0.44) | 0.51 | 0.23 | (-0.17, 0.62) | 0.26 | -0.17 | (-0.62, 0.28) | 0.46 |
| Stavudine | 5% | 0.12 | (-0.20, 0.44) | 0.45 | -0.09 | (-0.49, 0.32) | 0.67 | -0.25 | (-0.70, 0.20) | 0.27 |
| Tenofovir | 24% | -0.08 | (-0.25, 0.10) | 0.39 | -0.01 | (-0.22, 0.19) | 0.89 | -0.06 | (-0.29, 0.18) | 0.64 |
| Emtricitabine | 12% | -0.03 | (-0.26, 0.20) | 0.81 | 0.05 | (-0.22, 0.33) | 0.70 | -0.01 | (-0.32, 0.31) | 0.97 |
| NNRTIs | ||||||||||
| Efavirenz | 5% | 0.09 | (-0.26, 0.44) | 0.62 | -0.08 | (-0.49, 0.33) | 0.71 | -0.11 | (-0.56, 0.35) | 0.64 |
| Nevirapine | 14% | 0.08 | (-0.12, 0.29) | 0.43 | -0.06 | (-0.31, 0.20) | 0.65 | -0.12 | (-0.41, 0.17) | 0.41 |
| PIs | ||||||||||
| Atazanavir | 8% | -0.02 | (-0.30, 0.26) | 0.89 | 0.19 | (-0.15, 0.53) | 0.27 | -0.06 | (-0.45, 0.33) | 0.76 |
| Nelfinavir | 43% | 0.02 | (-0.13, 0.17) | 0.76 | -0.01 | (-0.20, 0.18) | 0.92 | 0.02 | (-0.19, 0.22) | 0.88 |
| Lopinavir/Ritonavir | 27% | -0.03 | (-0.19, 0.14) | 0.76 | -0.12 | (-0.32, 0.08) | 0.25 | -0.06 | (-0.29, 0.18) | 0.62 |
|
| ||||||||||
|
1st Trimester Exposure (only associations with P ≤ 0.10 for at least one parameter)
| ||||||||||
| cARV | 49% | 0.12 | (-0.03, 0.27) | 0.12 | 0.03 | (-0.15, 0.22) | 0.72 | -0.22 | (-0.42, -0.01) | 0.036 |
| NRTIs | ||||||||||
| Lamivudine | 42% | 0.10 | (-0.06, 0.25) | 0.21 | 0.06 | (-0.12, 0.25) | 0.50 | -0.17 | (-0.38, 0.03) | 0.10 |
| Tenofovir | 14% | -0.00 | (-0.21, 0.21) | 0.99 | -0.01 | (-0.26, 0.25) | 0.96 | -0.32 | (-0.60, -0.03) | 0.029 |
| Emtricitabine | 7% | 0.06 | (-0.24, 0.36) | 0.70 | -0.02 | (-0.38, 0.33) | 0.90 | -0.40 | (-0.80, -0.00) | 0.049 |
| PIs | ||||||||||
| Lopinavir/Ritonavir | 11% | -0.00 | (-0.23, 0.22) | 0.98 | -0.11 | (-0.39, 0.16) | 0.42 | -0.40 | (-0.72, -0.09) | 0.013 |
ARV = antiretroviral, BMI = body mass index, cARV = combination antiretroviral, CI = confidence interval, NRTI = nucleoside reverse transcriptase inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor, PI = protease inhibitor
Each echocardiographic parameter was modeled separately in an adjusted linear regression model comparing those with the exposure of interest to unexposed (to that ARV regimen or drug), with adjustment for covariates as follows: mother’s use of illicit drugs during pregnancy and child’s sex (LV ejection fraction); child’s race and mother’s use of illicit drugs during pregnancy (LV fractional shortening); age at echo, mother’s use of alcohol during pregnancy, and child’s BMI (LV stress velocity index).
Structural left ventricular measures
(Table 4) Exposure to cARV, both overall and during the first trimester, was associated with lower LV dimension Z-scores. First trimester, but not overall, exposures to cARV were also associated with higher mean LV posterior wall thickness and mean thickness-to-dimension ratio Z-scores. Exposure to abacavir at any time during pregnancy was significantly associated with a lower mean LV dimension and a higher mean LV thickness-to-dimension ratio compared to those not exposed to abacavir. Exposure to atazanavir, both overall and in the first trimester, was associated with a higher mean LV posterior wall thickness Z-score, while overall exposure to nelfinavir or lopinavir/ritonavir was associated with lower mean LV posterior wall thickness Z-scores. First trimester exposure to nevirapine was associated with higher mean LV posterior wall thickness and mass Z-scores compared to children not exposed in utero to nevirapine.
Table 4.
Adjusted differences in structural echocardiographic parameter mean Z-scores with at least marginal significance for 411 HIV-exposed uninfected children in SMARTT exposed vs. unexposed in utero to specific ARV regimens or drugs
| ARV Exposure | Percent Exposed |
Left Ventricular Structural Echocardiographic Parametera
|
|||||
|---|---|---|---|---|---|---|---|
| Short Axis Dimension |
Posterior Wall Thickness |
Septal Thickness | Mass | Wall Stress | Thickness-to- Dimension Ratio |
||
|
| |||||||
| Mean (95% CI), P value |
Mean (95% CI), P value |
Mean (95% CI), P value |
Mean (95% CI), P value |
Mean (95% CI), P value |
Mean (95% CI), P value |
||
|
ANYTIME DURING PREGNANCY
| |||||||
| cARV | 95% | -0.44 (-0.85,-0.02) 0.040 | 0.05 (-0.34,0.44) 0.82 | 0.14 (-0.23,0.50) 0.46 | -0.16 (-0.54,0.23) 0.43 | -0.12 (-0.63,0.39) 0.65 | 0.14 (-0.25,0.54) 0.47 |
| Nucleoside Reverse Transcriptase Inhibitor (NRTIs) | |||||||
| Abacavir | 28% | -0.22 (-0.41,-0.02) 0.031 | 0.18 (-0.01,0.36) 0.061 | 0.02 (-0.16,0.19) 0.84 | -0.09 (-0.27,0.10) 0.36 | -0.16 (-0.40,0.08) 0.19 | 0.23 (0.06,0.41) 0.010 |
| Didanosine | 6% | -0.11 (-0.49,0.27) 0.59 | 0.20 (-0.18,0.58) 0.30 | 0.05 (-0.30,0.40) 0.78 | -0.03 (-0.41,0.35) 0.89 | -0.48 (-0.96,0.00) 0.051 | 0.26 (-0.10,0.62) 0.15 |
| Non-Nucleoside Reverse Transcriptase Inhibitor (NRTIs) | |||||||
| Nevirapine | 14% | -0.01 (-0.26,0.24) 0.93 | 0.21 (-0.03,0.45) 0.084 | 0.22 (-0.00,0.45) 0.052 | 0.24 (-0.00,0.47) 0.053 | -0.16 (-0.47,0.16) 0.33 | 0.12 (-0.11,0.35) 0.31 |
| Protease Inhibitors (PIs) | |||||||
| Atazanavir | 8% | -0.38 (-0.71,-0.04) 0.030 | 0.47 (0.15,0.78) 0.004 | -0.14 (-0.44,0.15) 0.34 | -0.15 (-0.46,0.17) 0.36 | -0.11 (-0.52,0.31) 0.62 | 0.61 (0.30,0.91) <0.001 |
| Nelfinavir | 43% | -0.03 (-0.21,0.16) 0.76 | -0.21 (-0.38,-0.04) 0.017 | -0.10 (-0.26,0.06) 0.22 | -0.12 (-0.29,0.05) 0.16 | 0.07 (-0.15,0.30) 0.51 | -0.21 (-0.38,-0.04) 0.017 |
| Lopinavir/RTV | 27% | 0.10 (-0.10,0.30) 0.33 | -0.19 (-0.38,0.00) 0.055 | -0.13 (-0.31,0.05) 0.16 | -0.12 (-0.31,0.07) 0.21 | 0.02 (-0.23,0.27) 0.88 | -0.16 (-0.34,0.02) 0.083 |
|
| |||||||
|
FIRST TRIMESTER EXPOSURES
| |||||||
| cARV | 49% | -0.19 (-0.37,-0.01) 0.040 | 0.20 (0.03,0.37) 0.023 | -0.02 (-0.18,0.14) 0.81 | -0.02 (-0.19,0.15) 0.81 | -0.27 (-0.49,-0.05) 0.016 | 0.18 (0.02,0.35) 0.030 |
| Nucleoside Reverse Transcriptase Inhibitor (NRTIs) | |||||||
| Zidovudine | 39% | -0.15 (-0.33,0.04) 0.12 | 0.19 (0.02,0.37) 0.031 | 0.02 (-0.15,0.18) 0.85 | 0.01 (-0.16,0.19) 0.91 | -0.27 (-0.49,-0.05) 0.017 | 0.14 (-0.04,0.31) 0.12 |
| Lamivudine | 42% | -0.10 (-0.29,0.08) 0.27 | 0.18 (0.01,0.35) 0.039 | -0.01 (-0.17,0.15) 0.91 | 0.04 (-0.13,0.21) 0.63 | -0.21 (-0.43,0.01) 0.068 | 0.11 (-0.06,0.28) 0.20 |
| Abacavir | 14% | -0.14 (-0.40,0.12) 0.28 | 0.22 (-0.02,0.46) 0.075 | -0.01 (-0.23,0.22) 0.96 | -0.03 (-0.27,0.21) 0.82 | -0.25 (-0.57,0.07) 0.13 | 0.23 (-0.00,0.45) 0.052 |
| Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTIs) | |||||||
| Nevirapine | 10% | -0.08 (-0.38,0.21) 0.58 | 0.34 (0.06,0.62) 0.019 | 0.24 (-0.02,0.50) 0.076 | 0.28 (0.00,0.56) 0.049 | -0.15 (-0.52,0.21) 0.41 | 0.23 (-0.04,0.50) 0.094 |
| Protease Inhibitors (PIs) | |||||||
| Atazanavir | 5% | -0.37 (-0.77,0.02) 0.064 | 0.64 (0.27,1.01) 0.001 | -0.09 (-0.44,0.27) 0.63 | -0.00 (-0.38,0.37) 0.99 | -0.15 (-0.65,0.35) 0.55 | 0.72 (0.37,1.07) <0.001 |
| Nelfinavir | 19% | -0.11 (-0.35,0.12) 0.34 | -0.05 (-0.28,0.17) 0.64 | -0.18 (-0.39,0.03) 0.093 | -0.13 (-0.35,0.09) 0.26 | -0.02 (-0.30,0.25) 0.87 | -0.12 (-0.34,0.11) 0.31 |
| Lopinavir/RTV | 11% | -0.02 (-0.29,0.26) 0.91 | -0.05 (-0.32,0.21) 0.70 | -0.12 (-0.36,0.13) 0.36 | -0.13 (-0.39,0.13) 0.33 | -0.30 (-0.65,0.05) 0.094 | -0.02 (-0.27,0.23) 0.86 |
ARV = antiretroviral, BMI = body mass index, LV = left ventricular
Each echocardiographic parameter was modeled separately in an adjusted linear regression model comparing those with the exposure of interest to unexposed (to that ARV regimen or drug), with adjustment for covariates as follows: child’s race, child’s sex, child’s BMI, and mother’s age at delivery (LV short axis dimension); mother’s alcohol use during pregnancy (LV posterior wall thickness); mother’s alcohol use during pregnancy and child’s sex (septal thickness); maternal alcohol use during pregnancy, child’s sex and child’s BMI (LV mass);age at echo, child’s sex, and mother’s age at delivery (LV wall stress); child’s race, mother’s alcohol use during pregnancy and child’s BMI (LV thickness-to-dimension ratio)
The results of a sensitivity analysis limited to children with in utero exposure to cARV (95% of the SMARTT HEU cohort) were generally similar to those of the original analysis with two exceptions: 1) overall ZDV exposure was associated with a significantly higher mean LV stress velocity index Z-score in the sensitivity analysis (P = 0.022) but not in the original analysis (P=0.10), and 2) overall exposure to abacavir was not significantly associated with the LV dimension Z-score in the sensitivity analysis (P=0.09) although it was in the original analysis (P = 0.041).
In addition to the associations of echocardiographic Z-scores with in utero ARV exposures, we also observed several maternal and fetal factors that were independently associated with echocardiographic measures among the HEU cohort. Tobacco and alcohol use during pregnancy were associated with significant decreases in the LV ejection fraction Z-score. Tobacco use during pregnancy was also associated with a 0.23 mean decrease in LV fractional shortening Z-score (P = 0.034). Both tobacco and alcohol use during pregnancy were associated with higher septal thickness Z-score (P=0.032 and P=0.019, respectively), and maternal alcohol use was associated with a 0.51 increase in mean LV mass Z-score (P=0.004). In contrast, there was no association observed between echocardiographic parameters and any pregnancy outcomes, maternal age at delivery, or maternal health status as reflected by CD4 and viral load measurements prior to delivery. Girls had significantly lower LV ejection fraction (adjusted mean difference vs. boys = -0.15, P=0.040) and significantly lower mean Z-scores for several structural parameters (LV dimension, posterior wall thickness, septal thickness, and mass). White and Hispanic children had significantly higher mean Z-scores for LV dimension as compared to non-white and non-Hispanic children, respectively.
DISCUSSION
We found no evidence of clinically significant cardiac toxicity associated with perinatal ARV exposure among HEU children 3-5 years of age. There were no statistically significant differences between the HEU (95% exposed to cARV) and the HIV-unexposed SMARTT cohorts in mean Z-scores for any echocardiographic parameter. However, we did observe some associations between specific ARV exposures and certain echocardiographic parameters within the HEU cohort. While the overall cardiac function of the HEU children was similar to that of the SMARTT HIV-unexposed cohort, there was significant variability within the HEU cohort in echocardiographic Z-scores which could be at least partially explained by ARV exposures. Both the SMARTT HEU and HIV-unexposed cohorts differed from the BCH reference cohort’s mean Z-scores of zero for some parameters. This observation may reflect the effects of differences between the SMARTT cohorts and the BCH reference cohort for factors such as race-ethnicity, income and education level, nutritional status, environmental exposures, and other demographic and lifestyle characteristics. However, these differences would be unlikely to affect our observed associations of echocardiographic parameter with certain early prenatal cARV exposures in the HEU group.
As noted above, certain ARV exposures were associated with differences in mean Z-scores for echocardiographic parameters within the HEU group. We found significantly lower LV stress velocity index (a load-independent measure of LV function) in HEU children exposed in the first trimester to cARV and specific NRTIs (tenofovir, emtricitabine) or lopinavir/ritonavir, compared to HEU children not exposed to cARV or these specific agents. Exposure to cARV and specific NRTI and PI agents anytime during pregnancy was generally associated with lower LV dimension than lack of exposure to these regimens or agents, although this does not necessarily reflect a lower LV dimension than expected in comparison to the BCH reference cohort. Because the number of cardiac myocytes is fixed at birth, a small LV is a potential concern regarding the ability of the LV to meet the increased cardiovascular demands as these children grow into adulthood. Only longitudinal cardiac reassessment of this population can address this concern. First trimester exposures to cARV and to specific agents from all 3 ARV drug classes were associated with higherLV posterior wall thickness. All of the differences in Z-scores were less than 1 SD, and most were less than 0.5 SDs. Differences in echocardiographic Z-scores greater than 2 SD are typically considered pathologic, but the potential clinical significance of the smaller changes we observed are unknown.
The current findings of slightly lower LV function, lower dimension, and higher posterior wall thickness associated with cARV and specific ARV exposures, compared to children unexposed to cARV or these specific ARVs, could be consistent with a subclinical inflammatory response characterized by injury or death of cardiac myocytes along with an inflammatory infiltrate in the LV posterior wall and interventricular septum. These findings are found in other inflammatory heart conditions such as myocarditis [24-26]. These conclusions are further supported by a previous evaluation of cardiac and inflammatory biomarkers in the same SMARTT study population which could be consistent with a myocardial inflammatory process [27]. Since almost all mothers received ARVs during pregnancy, the relative contributions to a possible inflammatory response of prenatal exposure to ARVs vs. maternal HIV cannot be addressed in this analysis. Future studies of cardiac biomarkers may help identify HEU children who require further cardiac evaluation including echocardiography. Innate immune activation has been proposed as one of the reasons for the increased cardiovascular risk and mortality seen in HIV-infected adults receiving long-term ARV therapy [28-31].
We also found that maternal alcohol and tobacco use during pregnancy were associated with lower LV function as well as higher septal thickness and LV mass, independent of the observed ARV associations. Maternal alcohol use during pregnancy has been associated with structural cardiac defects, long QT syndrome (a potential risk for sudden cardiac death), and heart muscle disease in both human and animal studies [32-37]. Prenatal tobacco exposure has been associated with inhibition of cardiac DNA synthesis, impaired vascular smooth muscle structure and function, and structural cardiac defects [37].
A descriptive comparison of the SMARTT cohort with the earlier CHAART I and P2C2 HEU studies showed echocardiographic findings which were generally consistent for the SMARTT and previous CHAART I studies (both conducted in the cARV era) in terms of direction, although not always of the same magnitude. Echocardiographic findings were generally more extreme in the P2C2 HEU cohort and sometimes in the opposite direction, compared to the SMARTT and CHARRT-I cohorts but these differences were not consistent across all echocardiographic parameters. The P2C2 HEU cohort was either ARV-unexposed or only ZDV-exposed in the perinatal period and generally had more extreme changes in echocardiographic parameters. This suggests that the cARV regimens taken by mothers in the SMARTT and CHAART I cohorts and the resulting decrease in maternal viral load or inflammatory mediators led not only to a marked reduction in MTCT of HIV but also to a healthier echocardiographic profile at age 3 to 5 years. However, there could be differences in non-cARV exposures in the SMAART and CHAART-I mothers, such as maternal health, lifestyle, or environmental exposures, compared to the P2C2 HEU mothers, which could contribute to the healthier echocardiographic profile in their young HEU children.
In the SMARTT study we found lower Z-scores for several echocardiographic parameters in girls compared to boys. In the CHAART I study, differences in several echocardiographic measures were greater in girls, as opposed to boys, when compared to the P2C2 comparison group [15]. In healthy children, there are no gender differences in echocardiographic Z-scores. The reason for these gender differences in echocardiographic measures in HEU children in the SMARTT and CHAART I studies is not clear.
Study limitations include that a single echocardiographic assessment per child does not allow evaluation of the trajectory or persistence of any cardiac changes associated with ARV exposures. This study design cannot differentiate between potential cardiac effects of perinatal exposure to HIV and perinatal exposure to specific ARV agents. Our comparison of the SMARTT and CHAART I ARV exposed cohorts to the P2C2 ARV-unexposed cohort suggests that potential cardiac effects of perinatal ARV exposure in the cARV era are less extreme than in the pre-cARV era. Alternatively, this may indicate that poorer HIV control in pre-cARV era mothers could explain the cardiac effect differences instead of, or in addition to, any ARV effects. Finally, the current analysis utilized an initial “predictive” screening analysis which is appropriate to identify potential safety signals, but cannot evaluate causal relationships. However, the large number of participants and inclusion of both internal (SMARTT HIV-unexposed) and external (Boston Children’s Hospital) comparison groups are key strengths of this study, although the BCH reference cohort differed in terms of racial distribution and possibly socioeconomic status from both the HEU and HIV-unexposed cohorts. The analyses were adjusted for known potential confounders. However, there is still the possibility of residual confounding from unmeasured covariates. Finally, since the SMARTT study was primarily a safety study, we did not adjust for multiple comparisons to minimize Type II errors. Due to the large number of comparisons, some associations could be due to chance and findings should be confirmed in further studies.
We found no evidence of clinically significant cardiac toxicity associated with perinatal ARV (mostly cARV) exposure in HEU children at 3 to 5 years of age compared to a demographically similar reference cohort of HIV-unexposed and apparently healthy children. However, the associations of subclinical differences in LV structure and function with specific in utero ARV exposures, particularly in the first trimester, suggest that these children should be longitudinally studied to determine if long-term deleterious cardiac effects emerge as they age. Such follow-up studies could inform the selection of optimal ARV regimens in pregnancy that will simultaneously prevent perinatal transmission of HIV and optimize long-term cardiac health in infants born to HIV-infected mothers.
Acknowledgments
Sources of Funding: National Institutes of Health (HD052102; HD052104).
Funding/Support: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). Data management services were provided by Frontier Science and Technology Research Foundation, and regulatory services and logistical support were provided by Westat, Inc. Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
We thank the children and families for their participation in PHACS and the individuals and institutions involved in the conduct of PHACS. The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2010: William Shearer, Mary Paul, Norma Cooper, Lynette Harris (Baylor College of Medicine); Murli Purswani, Emma Stuard, Anna Cintron (Bronx Lebanon Hospital Center); Ana Puga, Dia Cooley, Doyle Patton, Deyana Leon (Children’s Diagnostic & Treatment Center); Richard Rutstein, Carol Vincent, Nancy Silverman (Children’s Hospital of Philadelphia); Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter (Children’s Memorial Hospital); Andrew Wiznia, Marlene Burey, Molly Nozyce (Jacobi Medical Center); William Borkowsky, Sandra Deygoo, Helen Rozelman (New York University School of Medicine); Katherine Knapp, Kim Allison, Megan Wilkins (St. Jude Children’s Research Hospital); Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera (San Juan Hospital/Department of Pediatrics); Hermann Mendez, Ava Dennie, Susan Bewley (SUNY Downstate Medical Center); Sharon Nachman, Margaret Oliver, Helen Rozelman (SUNY Stony Brook); Russell Van Dyke, Karen Craig, Patricia Sirois (Tulane University Health Sciences Center); Marilyn Crain, Newana Beatty, Dan Marullo (University of Alabama, Birmingham); Stephen Spector, Jean Manning, Sharon Nichols (University of California, San Diego); Elizabeth McFarland, Emily Barr, Robin McEvoy (University of Colorado Denver Health Sciences Center); Mobeen Rathore, Kristi Stowers, Ann Usitalo (University of Florida/Jacksonville); Kenneth Rich, Delmyra Turpin, Renee Smith (University of Illinois, Chicago); Douglas Watson, LaToya Stubbs, Rose Belanger (University of Maryland, Baltimore); Arry Dieudonne, Linda Bettica, Susan Adubato (University of Medicine and Dentistry of New Jersey); Gwendolyn Scott, Claudia Florez, Elizabeth Willen (University of Miami); Toinette Frederick, Mariam Davtyan, Maribel Mejia (University of Southern California); Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio (University of Puerto Rico Medical Center).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the US Department of Health and Human Services.
Footnotes
Author Contributions: S. E. L., P. L.W., B. Z., J.W., and G.R.S. designed the study. P.L.W. and B.Z. analyzed the data. S.E.L., P.L.W., B.Z., J.D.W., R.V.D., G.R.S., G.K.S., J.R.K., L.M.M. drafted the manuscript. All authors contributed to interpreting the data, critically revising the manuscript and approved the final version.
The initial results of this study were presented by Paige L. Williams as an oral presentation at the International Workshop on HIV Observational Databases on March 25, 2010 and by Steven E. Lipshultz as a poster presentation at the American Heart Association Scientific Session in Chicago, IL on November 15, 2010 but the results of this study have not been submitted to any other journal for publication.
Conflicts of Interest: All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
None of the authors have any conflicts of interest or disclaimers or have received compensation regarding this study.
References
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;33:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Diaz C, Hayani K, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- Griner R, Williams PL, Read JS, Seage GR, 3rd, Crain M, Togev R, et al. In utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS Patient Care STD. 2011;25:385–394. doi: 10.1089/apc.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Nguyen V, Ewings EL, Ceresa A, Shaw JA, St Claire MC, et al. Mitochondrial toxicity in fetal Erythrocebus patas monkeys exposed transplacentally to zidovudine plus lamivudine. AIDS Res Hum Retroviruses. 2004;20:91–100. doi: 10.1089/088922204322749530. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Erhart SW, Paik CY, St Claire MC, Nagashima K, Skopets B, et al. Fetal mitochondrial heart and skeletal muscle damage in Erthrocebus patas monkey exposed in utero to 3’-azido-3’-deoxythymidine. AIDS Res Hum Retroviruses. 2000;16:635–644. doi: 10.1089/088922200308864. [DOI] [PubMed] [Google Scholar]
- Lewis W. Mitochondrial toxicity of antiviral nucleosides used in AIDS: insights derived from toxic changes observed in tissues rich in mitochondria. In: Lipshultz SE, editor. Cardiology in AIDS. New York, NY: Chapman & Hall; 1998. pp. 317–329. [Google Scholar]
- Marin-Garcia J, Goldenthal MJ, Ananthakrishnan R, Pierpont ME, Fricker FJ, Lipshultz SE, et al. Specific mitochondrial DNA deletions in idiopathic dilated cardiomyopathy. Cardiovasc Re. 1996;31:306–313. [PubMed] [Google Scholar]
- Marin-Garcia J, Goldenthal MJ, Ananthakrishnan R, Pierpont ME, Fricker FJ, Lipshultz SE, et al. Mitochondrial function in children with idiopathic dilated cardiomyopathy. J Inherit Metab Dis. 1996;19:309–312. doi: 10.1007/BF01799259. [DOI] [PubMed] [Google Scholar]
- Brogly SB, Ylitalo N, Mofenson LM, Oleske J, Van Dyke R, Crain MJ, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- Crain MJ, Chernoff MC, Oleske JM, Brogly SB, Malee KM, Borum PR, et al. Possible mitochondrial dysfunction and its association with antiretroviral therapy use in children perinatally infected with HIV. J Infect Dis. 2010;202:291–301. doi: 10.1086/653497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR, 3rd, et al. The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS. 2012;26:2027–2037. doi: 10.1097/QAD.0b013e3283578bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. Absence of cardiac toxicity of zidovudine in infants. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 2000;343:759–766. doi: 10.1056/NEJM200009143431102. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Shearer WT, Thompson B, Rich KC, Cheng I, Orav EJ, et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study) J Am Coll Cardiol. 2011;57:76–85. doi: 10.1016/j.jacc.2010.08.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- P2C2 HIV Study Group. The pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus (P2C2 HIV) infection study: design and methods. J Clin Epidemiol. 1996;49:1285–1294. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne JE, Shearer WT, Thompson B, Orav EJ, Starc TJ, Coaln SD, et al. Cardiovascular outcomes of pediatric seroverters perinatally exposed to HAART: design of a longitudinal clinical study. Cardiovasc Toxicol. 2004;4:187–197. doi: 10.1385/ct:4:2:187. [DOI] [PubMed] [Google Scholar]
- Williams PL, Seage GR, 3rd, Van Dyke RB, et al. A trigger-based design for evaluating the safety of in utero antiviral exposure in uninfected children of HIV-infected mothers. 2012. Am J Epidemiol. 2012;175:950–961. doi: 10.1093/aje/kwr401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschengrau A, Seage GR., 3rd . Essentials of Epidemiology in Public Health. Third ed. Sudbury, MA: Jones and Bartlett; 2014. [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Greenland S, Robins JM. Empirical-Bayes adjustments for multiple comparisons are sometimes useful. Epidemiology. 1991;2:244–51. doi: 10.1097/00001648-199107000-00002. [DOI] [PubMed] [Google Scholar]
- Colan SD, Borow KM, Neumann A. Left ventricular end-systolic wall stress-velocity of fiber shortening relation: a load independent index of myocardial contractility. J Am Coll Cardiol. 1984;4:715–724. doi: 10.1016/s0735-1097(84)80397-6. [DOI] [PubMed] [Google Scholar]
- Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl U, Schultheiss HP. Viral myocarditis: diagnosis, aetiology and management. Drugs. 2009;69:1287–1302. doi: 10.2165/00003495-200969100-00001. [DOI] [PubMed] [Google Scholar]
- Cocker M, Friedrich MG. Cardiovascular magnetic responance of myocarditis. Curr Cardiol Rep. 2010;12:82–89. doi: 10.1007/s11886-009-0077-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JD, Williams PL, Leister E, Zeldow B, Shearer WT, Colan SD, et al. Cardiac biomarkers in HIV-exposed uninfected children. AIDS. 2013;27:1099–1108. doi: 10.1097/QAD.0b013e32835cf21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity [Abstract] BM. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–529. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann T. QT prolongation in the newborn and maternal alcoholism. Cardiol Young. 2004;14:565–566. doi: 10.1017/S1047951104005177. [DOI] [PubMed] [Google Scholar]
- Krasemann T, Klingebiel S. Influence of chronic intrauterine exposure to alcohol on structurally normal hearts. Cardiol Young. 2007;17:185–188. doi: 10.1017/S1047951107000224. [DOI] [PubMed] [Google Scholar]
- Löser H, Pfefferkorn JR, Themann H. Alcohol in pregnancy and fetal heart damage. Klin Padiatr. 1992;204:335–339. doi: 10.1055/s-2007-1025368. [DOI] [PubMed] [Google Scholar]
- Adickes ED, Mollner TJ, Makoid MC. Teratogenic effects of ethanol during hyperplastic growth in cardiac myocyte cultures. Alcohol Clin Exp Res. 1993;17:988–992. doi: 10.1111/j.1530-0277.1993.tb05653.x. [DOI] [PubMed] [Google Scholar]
- Wold LE, Norby FL, Hintz KK, Colligan PB, Epstein PN, Ren J. Prenatal ethanol exposure alters ventricular myocyte contractile function in the offspring of rats: influence of maternal Mg2R supplementation. Cardiovasc Toxicol. 2001;1:215–224. doi: 10.1385/ct:1:3:215. [DOI] [PubMed] [Google Scholar]
- Mone SM, Gillman MW, Miller TL, Herman EH, Lipshultz SE. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004;113(Suppl 4):1058–1069. [PubMed] [Google Scholar]


