Figure 6.
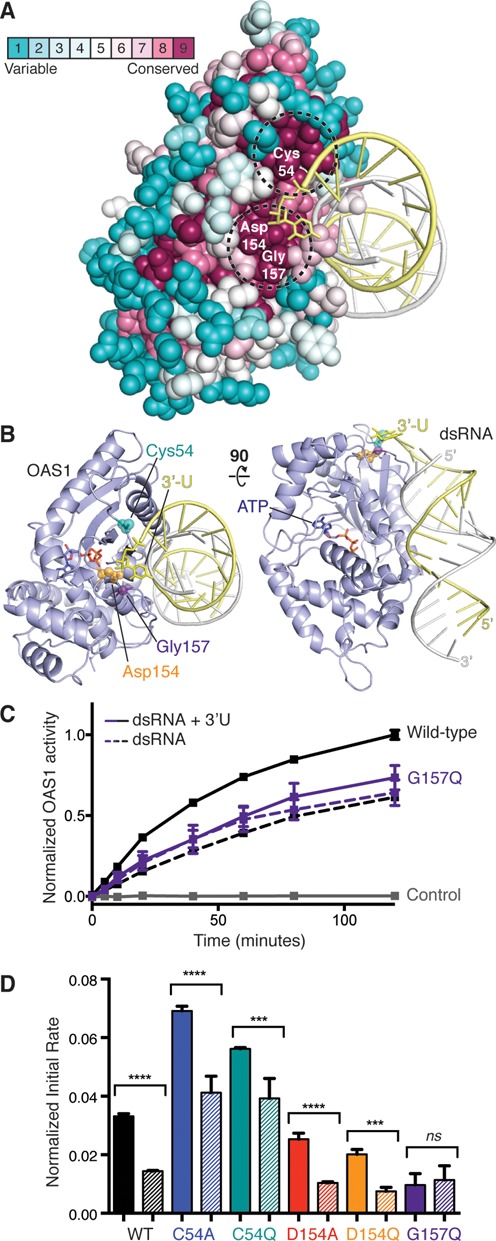
OAS1 G157 is a critical mediator of 3′-ssPy motif action. (A) Consurf analysis of OAS1 run using the X-ray crystal structure of the human protein (PDB ID: 4IG8) determined in complex with the 18-bp dsRNA duplex. Two highly conserved patches (dashed circles) are located adjacent to the approximate position of the modeled 3′-ssPy motif. (B) Two orthogonal views of the OAS1-dsRNA structure with mutated protein residues and modeled 3′-ssPy motif highlighted. (C) Chromogenic assay showing the effect of the 3′-ssPy on wild-type and G157Q mutant OAS1 activity. (D) Comparison of initial rate of reaction for wild-type and mutant OAS1 proteins activated by the 18-bp dsRNA duplex with (solid bar) or without (striped bar) an additional 3′-end single-stranded uridine residue (3′-ssPy motif). One-way ANOVA: P ≤ 0.0001 (****), P between 0.0001 and 0.001 (***) and not significant (ns; P ≥ 0.05). In panels (C–D), data are normalized to wild-type OAS1 activation by 18-bp dsRNA with the 3′-ssPy motif.
