Fig. 4.
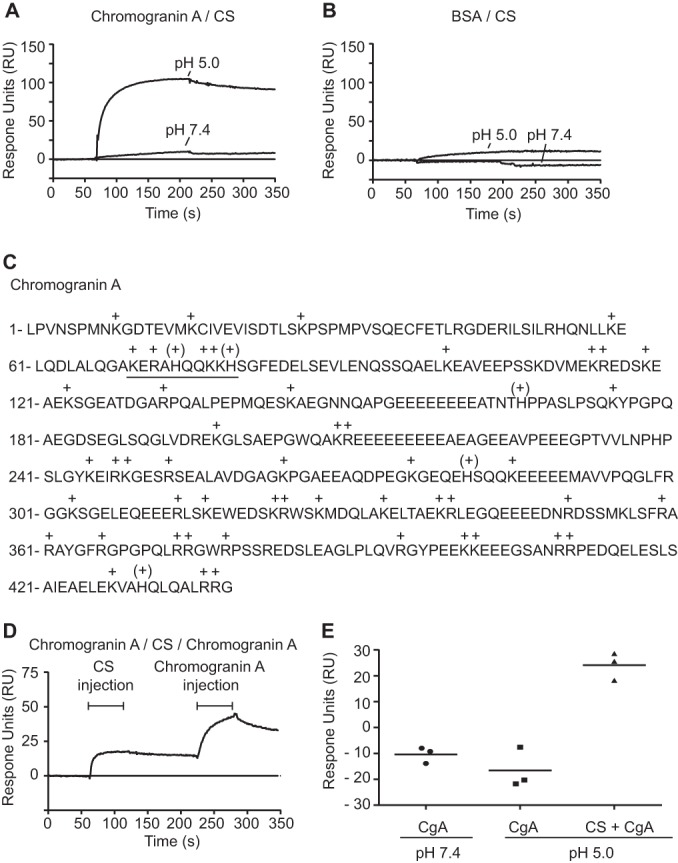
Complex formation of Chromogranin A and CS under mild acidic conditions. A–B, CgA core protein A, and BSA B, were immobilized onto a Biacore CM5 sensor chip (2000 RU) and CS (5 μm) was allowed to interact with the surfaces at neutral (pH 7.4) and under mild acidic conditions (pH 5.0). C, CgA amino acid sequence where histidine “(+)” and other basic amino acids “+” are marked. A basic peptide sequence likely to interact with negatively charged CS-chains is underlined. D, Sequential injection of CS (5 μm) and CgA (15 nm) over immobilized CgA (1000 RU) at pH 5.0 indicates CgA-CS-CgA complex formation. E, In contrast, CgA injected directly over immobilized CgA without any prior CS injection did not display any binding, neither at pH 5.0 nor at pH 7.4. The increment in CgA-binding of the three different experiments is shown. The lines represent the mean value of three independent experiments. Notably, a slight decrease in signal was observed for CgA and CgA interaction at both pH-conditions. This effect was related to a stronger binding of the analyte to the reference channel than that of the ligand channel.
