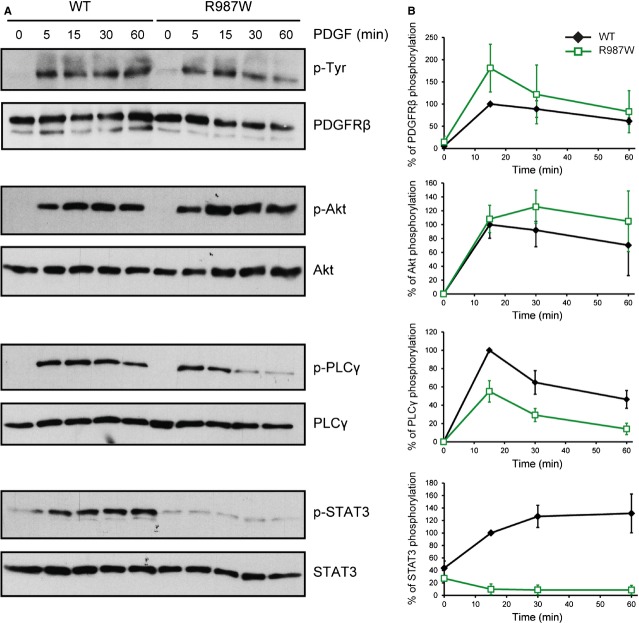
Fig. 3.
The R987W mutant does not activate STAT3. (A) Analysis of the phosphorylation and expression levels of the wild-type receptor and the R987W mutant as well as the phosphorylation of Akt, PLCγ and STAT3 upon ligand stimulation. HT-1080 cells stably expressing PDGFRβ WT or R987W were washed and starved overnight. Then, cells were treated or not with PDGF-BB (20 ng/ml) for 5, 15, 30 and 60 min. Cell lysates were analysed by western blot. After analysis of the phosphorylated protein, the membrane was re-probed with an antibody targeting the total amount of the corresponding protein. One representative experiment out of three is shown. (B) The percentage of PDGFRβ, Akt, PLCγ and STAT3 phosphorylation was quantified using the ImageJ software. The level of phosphorylation was normalized with the expression of the corresponding protein. The phosphorylation level of each protein was set to 100% for PDGFRβ WT-expressing cells stimulated with PDGF during 15 min. The average of three independent experiments is shown with SD (anova, P < 0.05 for p-PDGFRβ, not significant for p-Akt, P < 0.001 for p-PLCγ, P < 0.001 for p-STAT3).