Fig. 3.
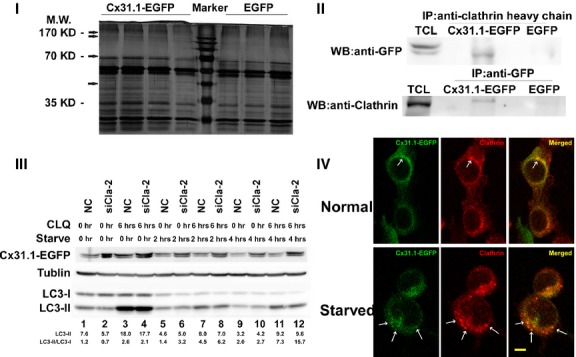
Cx31.1 could interact with clathrin, and clathrin might involve in the autophagy of Cx31.1-EGFP. (I) Characterization of proteins coimmunoprecipitated with Cx31.1. Silver-stained gel of protein after immunoprecipitation with anti-GFP. Bands of probable interactors of Cx31.1 were marked with arrows. H1299 cells stably expressing EGFP were used as a control. These bands were excised, in-gel digested and analysed by HPLC-MS on an LTQ-FT instrument using two consecutive stages of tandem MS. This experiment was repeated for three times. (II) Cx31.1-EGFP was coimmunoprecipitated together with clathrin. TCL, total cell lysate; Cx31.1-EGFP, protein coimmunoprecipitated from Cx31.1-EGFP-H1299 cells; EGFP, protein coimmunoprecipitated from H1299 cells stably expressing EGFP. The samples were coimmunoprecipitated with antibody against clathrin heavy chain, then blotted with antibody against GFP and vice versa. (III) Knockdown clathrin expression enhanced Cx31.1-EGFP levels. Cx31.1-EGFP-H1299 cells were treated with siCla-2 or in combination with CLQ under normal growth condition, as well as starved for 2 and 4 hrs. Immunoblots were performed with antibodies against GFP and LC3. Cx31.1-EGFP-H1299 cells were transiently transfected with siCla-2 for 48 hrs. Before the cells lysates were collected, cells were treated with or without 30 μM CLQ for 6 hrs. In the case of starvation, the cells were incubated with CLQ-containing growth medium for 4 hrs or 2 hrs, and then the growth media was replaced with HBSS containing 30 μM chloroquine to starve cells for 2 or 4 hrs respectively. Cx31.1-EGFP levels were enhanced after siCla-2 treatment with or without serum starvation. LC3 was used as an indicator for autophagy. Duplicated gel was made for the immunoblotting of β-tubulin. The relative amount of LC3-II and the ratio of LC3-II/LC3-I were indicated. (IV) Confocal images of colocalization of Cx31.1-EGFP and clathrin under normal growth condition and starvation. Arrows indicated puncta with both Cx31.1-EGFP fluorescence and anti-clathrin immunoreactivities. Scale bar, 10 μm.
