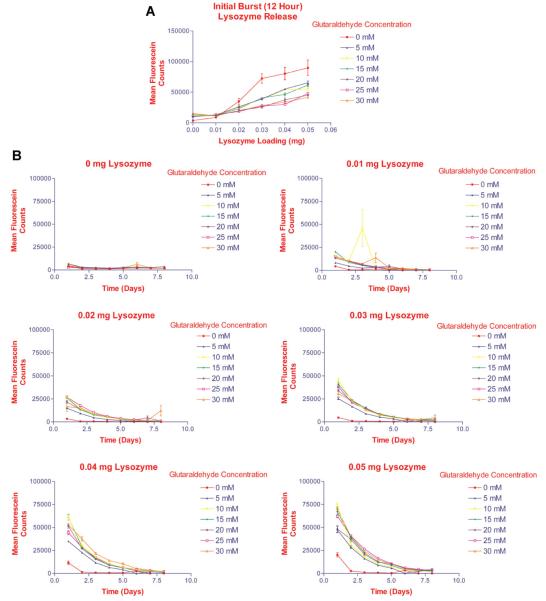
Fig. 1.
Determination of optimal glutaraldehyde concentration ranges required for crosslinking of the gelatin-based hydrogels. Gelatin was dissolved in PBS containing various concentrations of fluorescein-labeled lysozyme. The gelatin solutions were placed in 24-well plates and dried under laminar airflow for 8 h. Controls consisted of gelatin solution without lysozyme. The gelatin sheets were covered with glutaraldehyde solution at various concentrations, and allowed to crosslink at 4°C for 24 h. PBS was added to each well and the plates were placed in a 5% CO2 incubator at 37°C. After 12 h and then daily, the supernatants were aspirated completely, and replaced for 10 days to maintain sink conditions. Samples were analyzed by measuring fluorescence at an excitation wavelength (λ) = 495 nm and λemission = 515 nm. A: Initial burst release of lysozyme from hydrogels during first 12 h. Lysozyme release from the hydrogels correlated with the loading doses, and varied inversely to the degree of crosslinking of the hydrogels (glutaraldehyde concentration). B:Lysozyme release from hydrogels over 8 days suggested that crosslinking with 30 mM glutaraldehyde resulted in the most delayed release of lysozyme.