Abstract
Objective
Cognitive deficits are prominent in schizophrenia. Patients have an average score one standard deviation below normal on a broad spectrum of cognitive tests. It has been repeatedly noted, however, that 20%–25% of patients differ from this general pattern and score close to normal on neuropsychological testing. This study used brain morphometry to 1) identify brain abnormalities associated with more severe cognitive deficits and 2) help determine whether cognitively relatively intact patients perform better because they have less severe illness or because they have a different illness.
Method
Patients were assigned to a neuropsychologically near normal (N=21) subgroup if they scored within 0.5 standard deviation of healthy comparison subjects (N=30) on four tests of attention and verbal and nonverbal working memory, and to a neuropsychologically impaired (N= 54) group if they scored at least 1.0 standard deviation below that of comparison subjects. Subgroup assignments were confirmed with the California Verbal Learning Test and degraded-stimulus Continuous Performance Test. Volumes of ventricular compartments, hippocampus, amygdala, thalamus, cerebellum, and regional cortical gray and white matter were dependent variables. Differences among groups were evaluated by using linear mixed-model multivariate analyses with gender, age, and height as covariates.
Results
Both neuropsychologically near normal and neuropsychologically impaired patients had markedly smaller gray matter and larger third ventricle volumes than healthy comparison subjects. Only neuropsychologically impaired patients, however, had significantly smaller white matter and larger lateral ventricle volumes than healthy comparison subjects.
Conclusions
Although both neuropsychologically impaired and neuropsycho-logically near normal patients have marked neuropathology in their gray matter, the relative absence of white matter pathology in the neuropsychologically near normal group suggests the possibility of differences in the disease process.
Cognitive deficits are a central and prominent aspect of the pathophysiology and phenotype of schizophrenia (see reference 1 for a meta-analysis). Deficits are particularly marked in sustained attention, verbal memory, and manipulation of information in working memory, but the deficits extend to nearly all aspects of cognitive function, typically producing performance levels that are more than one standard deviation below the norm (1). In this context, it is noteworthy that some patients have scores that are normal, or close to normal, on many or all tests of cognition. This neuropsychologically near normal (NPNN) subgroup comprises 20%–25% of patients across multiple studies (2–9).
The pathophysiological significance of being NPNN is unclear. It is possible that some of these patients would have had superior abilities if not for their illness and that therefore they differ from patients who are cognitively more impaired only in their premorbid capabilities but not in the nature of their disease processes themselves. It seems improbable, however, that the 20%–25% of patients who have relatively intact cognition in typical outpatient samples would all have been destined to have cognitive abilities one to two standard deviations above the norm if they had not become ill, as that is a disproportionately large percentage of people to have cognitive abilities that far above the population norm. The differing cognitive abilities in those patients who are NPNN compared with those who are neuropsychogically impaired (NPI) could arise from differences in the severity of a single disease process. Consistent with this possibility, patients who have generally intact cognitive abilities may have more isolated deficits in certain cognitive domains (10), and they often have significant problems in real-world functioning (9). Yet another possibility is that NPNN and NPI patients differ in their cognitive abilities because they have illnesses that differ fundamentally in their underlying pathogenesis and consequently also in their effects on brain structure and function. Consistent with this possibility, NPI and NPNN patients have been found to differ in symptom patterns, with NPI patients having a higher ratio of negative to positive symptoms and an earlier age of illness onset (e.g., reference 7). Currently available data do not allow us to determine whether the differences in cognition across these two groups reflect quantitative differences in premorbid abilities, quantitative differences in illness severity, or qualitative differences in the underlying disease processes.
The present study approached the question of whether NPNN and NPI result from differing severity versus differing neurobiological subtype of illness by comparing NPNN and NPI patients on detailed measures of regional brain volumes of gray and white matter across the cerebrum. We evaluated two hypotheses. The first was that NPNN patients have morphological abnormalities qualitatively similar to, but quantitatively more limited than, those in NPI patients, which would suggest the presence of a milder form of illness. The second was that NPI patients have qualitatively distinct patterns of volumetric abnormalities that distinguish them from NPNN patients. Evidence of distinct patterns of anatomical abnormalities would suggest the presence of differing disease processes in the two groups or at least the presence of a differing stage of illness.
Experimental Methods
Subjects
Eighty-one symptomatic but stable outpatients who met DSMIV criteria for schizophrenia and 30 healthy comparison subjects participated after providing written informed consent. All but three patients had been in treatment for more than 5 years, most had been hospitalized more than three times (none had been hospitalized within the 3 months preceding study), none had abused substances for at least 60 days, and all had been taking their current medications for at least 30 days. Healthy subjects were without a history of axis I disorders, heavy substance use within the last 5 years, or neurological illness. Clinical symptoms at the time of study were assessed by doctoral-level psychologists with established interrater reliability using the Positive and Negative Symptom Scale for Schizophrenia (11).
Definition of Subgroups
NPNN and NPI patients were identified on the basis of two verbal and two nonverbal serial position working memory tasks (12, 13). Test stimuli were words, easily named sounds (e.g., telephone ringing), bird songs, or snowflake designs. Patients with schizophrenia have been shown repeatedly in previous studies to perform poorly on these tasks (12–14). Moreover, deficits in working memory in general and verbal memory in particular, are among the most consistent and robust cognitive deficits in schizophrenia (e.g., reference 15). Thus, both the verbal and nonverbal tests identify patients with wide-ranging deficits, whereas the verbal tests are particularly sensitive in identifying patients who have significant but more narrowly defined deficits. To validate this method for designating patients as NPNN or NPI, in the first 46 patients (14 NPNN and 32 NPI) and 22 healthy subjects enrolled, cognition was also evaluated using two additional tests that have been widely used in demonstrations of cognitive deficits in patients with schizophrenia, the California Verbal Learning Test (16) and a degraded stimulus version of the Continuous Performance Task (17).
Twenty-one patients were assigned to the NPNN group, based on overall scores on the four tests within 0.5 standard deviation of the mean score of healthy subjects. Fifty-four patients were assigned to the NPI group based on overall scores that were >1.0 standard deviation below the mean of the healthy comparison subjects. To improve the accuracy of correct subgroup assignment, six patients whose overall scores were between 0.5 and 1.0 standard deviations below the mean of the comparison subjects were excluded. Although these boundary definitions were selected a priori using commonly accepted criteria for normal (i.e., <0.5 standard deviation from comparison means) and abnormal (>1.0 standard deviation below the comparison mean), post hoc inspection showed that the distribution of scores from all patients (including the six excluded from subgroup assignment) was consistent with subpopulations defined by these boundaries. The distribution was nonnormal by the Shapiro Wilk test (p=0.03), with a major peak at 1.85 standard deviations below the mean of healthy comparison subjects and a second peak at exactly the mean of the healthy comparison subjects. Assignment of 28% of the patients to the NPNN group is similar to the proportion shown in previous studies to have either normal or close to normal cognition (2–9).
NPNN and NPI patients were similar both clinically and demographically (Table 1). The NPI group contained a higher percentage of women. Although not statistically significant, NPNN patients and their parents were somewhat better educated than NPI patients and their parents. Both patient groups were somewhat less educated than the healthy subjects, and the healthy comparison group contained fewer men and fewer African Americans. Similar percentages of NPNN and NPI patients were receiving atypical antipsychotic medications, although more NPI patients were also receiving typical antipsychotics. Patients receiving the typical antipsychotics did not differ in overall memory scores from those receiving only atypical antipsychotics (p>0.65). More NPI patients were receiving mood stabilizers and more NPNN patients were receiving antidepressant medications, but these differences were not associated with differences in symptom severity at the time of assessment or with the presence of comorbid diagnoses.
TABLE 1.
Demographic Characteristics of the Study Groups
| Patient Subgroups |
||||||
|---|---|---|---|---|---|---|
| Variable | Neuropsychologically Near Normal (N=2l) | Neuropsychologically Impaired Group (N=54) | Healthy Comparison Subjects (N=30) | |||
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 39.5 | 9.1 | 42.6 | 9.6 | 37.5 | 11.0 |
| Education (years) | 13.8 | 2.2 | 12.4 | 2.9 | 14.8 | 2.4 |
| Mother's education (years) | 13.6 | 3.7 | 12.1 | 3.5 | — | |
| Father's education (years) | 14.1 | 3.6 | 13.6 | 5.0 | — | |
| Duration of illness (years) | 17.0 | 7.9 | 18.9 | 8.7 | — | |
| Age of onset (years) | 23.0 | 5.0 | 23.0 | 7.8 | — | |
| Age first hospitalized (years) | 22.2 | 5.3 | 23.6 | 7.6 | — | |
| Positive and Negative Symptom Scale Ratings | — | |||||
| Positive | 14.1 | 5.7 | 15.1 | 6.0 | ||
| Negative | 16.4 | 6.7 | 15.6 | 5.2 | ||
| General | 30.9 | 12.5 | 31.7 | 8.8 | ||
| California Verbal Learning Test total | 44.7 | 11.9 | 36.0 | 9.7 | 52.6 | 12.9 |
| Degraded Stimulus Continuous Performance Test (d prime) | 2.1 | 1.0 | 1.5 | 1.0 | 2.0 | 1.0 |
| % | % | % | ||||
|---|---|---|---|---|---|---|
| Male | 85.7 | 63.0 | 43.3 | |||
| Ethnicity | ||||||
| African American | 38.1 | 38.0 | 6.7 | |||
| Caucasian | 57.1 | 56.0 | 83.3 | |||
| Hispanic | 0.0 | 6.0 | 3.3 | |||
| Other | 4.8 | 0.0 | 6.7 | |||
| Medication type | — | |||||
| Atypical antipsychotics | 72.2 | 59.1 | ||||
| Typical neuroleptics | 16.7 | 15.9 | ||||
| Both | 5.6 | 20.5 | ||||
| None | 5.6 | 4.5 | ||||
| Medication type (including both) | — | |||||
| % taking atypicals | 77.8 | 79.6 | ||||
| % taking typicals | 22.3 | 36.4 | ||||
| Mood stabilizers | 27.8 | 48.7 | — | |||
| Antidepressants | 38.9 | 23.1 | — |
Assessment of Brain Structure
Magnetic resonance images (MRIs) were acquired using a single 1.5 T GE Signa LS MRI System (Milwaukee) and a fast spoiled gradient-echo sequence (TR=24 msec, TE=5 msec, 256×192 matrix, field of view=30 cm, two excitations, slice thickness=1.2 mm, no skip, 124 sagittal slices). Analyses were performed on 10 workstations using ANALYZE 8.0 (Rochester, MN). Before region definitions, large-scale variations in image intensity due to refractive index coil and other inhomogeneities were removed. Extracerebral tissues were removed with an isointensity contour function that thresholds cortical gray matter from overlying CSF. Connecting dura and fat were removed manually. The data set was resliced to Talairach standard orientation to correct for residual head rotation, tilt, or flexion/extension.
The grayscale values of “pure” representations of cortical gray matter (the cortical ribbon) and white matter were sampled bilaterally in frontal, temporal, occipital, and parietal regions using an 8×8=64 pixel array that was sufficiently large to provide statistical stability but small enough to avoid partial volume effects from other tissue types. These four values were averaged for each tissue type. A global threshold, calculated as the average of mean gray matter and white matter values, was invoked to provide an initial rough classification of gray and white matter. This classification was then hand edited in all three views, primarily to eliminate subcortical gray matter and rims of ventricles (partial volumed white matter and ventricular CSF that is labeled as gray matter in most segmentation algorithms) from the tissue assigned to cortical gray matter. White matter was defined by subtraction of all other structures (cortical gray, subcortical gray, and ventricular CSF) from the isolated cerebrum (Figure 1).
FIGURE 1. Cortical Segmentationa.
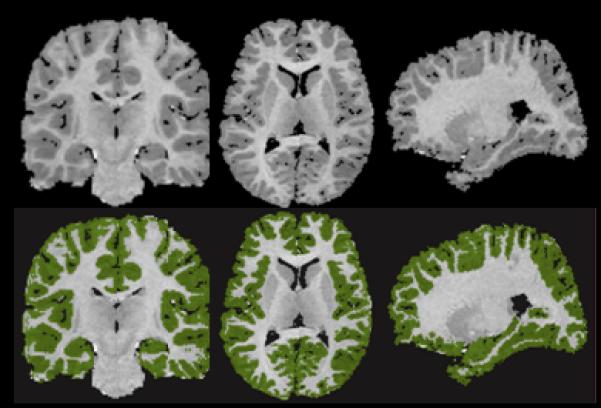
a Left: coronal; middle: axial; right: parasagittal views. Top: original gray scale image; bottom: segmented cortical gray matter (green). Images are in standard orientation.
Ventricles were defined with an isointensity contour function and manual editing. The third and fourth ventricles were isolated and lateral ventricles then divided into three sections—frontal horns, midbody, and occipital horns—using coronal planes passing through the anterior and posterior commissures. The temporal horn was separated from the lateral bodies with an axial plane containing the anterior commissure-posterior commissure line.
Using methods described previously (18, 19), the cerebral hemispheres were divided using a midsagittal curvilnear plane defined with a cubic spline fit to midline landmarks. The cerebrum was divided into eight regions within each hemisphere by the intersections of an axial plane containing the anterior and posterior commissures and three coronal planes: one tangent to the genu of the corpus callosum, one containing the anterior commissure, and one containing the posterior commissure. These three planes demarcated orbitofrontal, dorsal prefrontal, premotor, subgenual, sensorimotor, midtemporal, parietal, and inferior occipital subregions.
A total of four raters were used in these and the other volumetric analyses, with interrater reliability of the measurements assessed on 20 scans each, measured by the four raters and calculated with a two-way random-effects model. Intraclass correlation coefficients (ICC's) for cerebral subdivisions were all >0.98.
The thalamus was segmented by filtering the entire imaging volume with an anisotropic diffusion filter (unbiased, kappa=2, iterations=20) and then sampling grayscale values of the filtered thalamus and internal capsule throughout the entire three-dimensional extent of these structures, averaging the peaks for white matter and gray matter in that volume. An isointensity contour function at this particular threshold, grown from a seed within the thalamus, provided an initial definition of this structure that was then manually edited. The thalamus was distinguished from the hypothalamus by a line defining the hypothalamic sulcus on sagittal views. The ICC for thalamic definition was 0.91.
The amygdala and hippocampus were defined in the coronal plane with previously published algorithms (20, 21). ICC's were >0.85 for the amygdala and >0.90 for the hippocampus. Difficulty in identifying some key landmarks led to some missing data in three patients.
Statistical Analyses
Differences among patient subgroups and healthy subjects on the California Verbal Learning Test and degraded stimulus version of the Continuous Performance Test scores were evaluated in oneway analyses of variance (ANOVAs) with post hoc pairwise comparisons using Fisher's protected test of least squares difference.
Differences among the three groups in brain structure were evaluated with linear mixed models separately for cortical gray and white matter subregions, ventricles, hippocampus, amygdala, thalamus, and cerebellum. Gender, age, and height (to control for overall scaling effects) were included in the analytic models because each has known correlates in brain volume. Because of the small number of women, interactions between gender and group were considered unreliable and removed from the models. Significant main effects and interactions of group were followed up with Fisher's test: p values of <0.05 were used to identify group differences. The ability of the anatomic differences between the two patient groups to discriminate the groups was then evaluated with PROC CANDISC in SAS 9.1 (SAS Institute, Cary, NC), with covariance for age, gender, and height. All tests were two-tailed.
Results
Differences in Memory and Attention
ANOVAs of California Verbal Learning Test total scores revealed a significant effect of group (F=14.4, df=2, 65, p<0.0001). NPI patients performed significantly less well than did both the NPNN patients and the healthy comparison subjects. The NPNN patients performed less well than did the healthy subjects at a level of significance of p=0.07. The differences among groups approached significance on the degraded stimulus version of the California Verbal Learning Test (F=2.6, df=2, 61, p=0.08), with NPI patients tending toward lower scores than both NPNN patients (p= 0.06) and healthy subjects (p=0.08), and NPNN patients performing nonsignificantly better than healthy subjects.
Group Differences in Ventricular Size
The main effect of group was significant (F=4.2, df=2, 98, p=0.02), and the interaction between group and region approached significance (F=1.7, df=10, 495, p=0.09). Post hoc comparisons (Table 2) showed that the NPI group relative to comparison subjects had significantly greater volumes of all ventricular compartments (p=0.02 to p=0.0007). The NPNN patients, in contrast, did not differ significantly from the healthy comparison subjects in any compartments of the lateral ventricles (p=0.11–0.46). Similar to the NPI patients, NPNN patients had significantly larger third ventricles relative to comparison subjects (p=0.004) and showed a tendency for larger fourth ventricles (p=0.06).
TABLE 2.
Brain and Ventricular Volumes of Patient Subgroups and Healthy Comparison Subjects
| Subject Group | Cortex (mm3) |
Cortex (mm3) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue Type | Dorsolateral Prefrontal |
Premotor |
Sensorimotor |
Parietal |
Orbitol-frontal |
Subgenual |
Midtemporal |
Inferior Occipital |
|||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| Neuropsychologically near normal patients (N=21) | gray matter | 26502a | 796 | 26794a | 901 | 32008a | 977 | 75222a | 1953 | 5802a | 342 | 14712a | 514 | 18395a | 474 | 32646a | 1055 |
| white matter | 21662 | 771 | 39823 | 1187 | 55600 | 1377 | 85389 | 2464 | 3139 | 280 | 8171 | 400 | 21996 | 636 | 28413 | 1046 | |
| Neuropsychologically impaired patients (N=54) | gray matter | 25929a | 510 | 26677a | 580 | 31175a | 628 | 74261a | 1253 | 6273 | 217 | 15048a | 327 | 18690a | 305 | 32516a | 672 |
| white matter | 20452a | 473 | 37962a | 727 | 52473b | 842 | 78345b | 1505 | 2950a | 174 | 8022a | 246 | 21650 | 389 | 26303 | 641 | |
| Healthy comparison subjects (N=30) | gray matter | 30116 | 667 | 31928 | 756 | 36201 | 819 | 86310 | 1637 | 6778 | 286 | 17361 | 430 | 19927 | 398 | 35481 | 882 |
| white matter | 22992 | 650 | 41329 | 1001 | 55304 | 1161 | 86693 | 2078 | 3658 | 235 | 8949 | 336 | 22630 | 535 | 28225 | 883 | |
Patient subgroup differs significantly from healthy comparison subjects.
Patient subgroup differs significantly from healthy comparison subjects and other patient groups.
Group Differences in White Matter
The main effect of group (F=5.7, df=2, 98, p=0.005) and the interaction between group and region (F=1.8, df=14, 693, p=0.03) were significant. Post hoc comparisons (Table 2) showed that NPI patients had significantly smaller white matter volumes in relation to healthy comparison subjects in the dorsal prefrontal (p=0.001), premotor (p=0.006), sensorimotor (p=0.05), parietal-occipital (p=0.001), orbito-frontal (p=0.02), and subgenual (p=0.03) regions, and they tended toward less white matter in the inferior occipital region (p=0.07). Effect sizes ranged from –0.75 to –0.45, with confidence intervals supporting significance. In contrast, NPNN patients did not differ from healthy subjects in any region (p values=0.14–0.87). Effect sizes ranged from 0.05 to –0.42, with confidence intervals that spanned 0.00, indicating nonsignificance. Power estimates indicate that none of these effect sizes would have been significant if the NPNN sample had been the same size as the NPI sample. The NPI and NPNN groups differed significantly from each other in volumes of the sensorimotor (p=0.05) and parietal-occipital (p=0.01) regions, and they showed a tendency to differ in the inferior occipital region (p=0.09).
Group Differences in Cortical Gray Matter
The main effect of group (F=17.4, df=2, 98, p<0.000001) and interaction between group and region (F=3.8, df=14, 686, p<0.00001) were significant. Post hoc comparisons (Table 2) showed that both patient groups had significantly lower gray matter volumes than did healthy comparison subjects in all regions except the orbitofrontal cortex, where the NPNN (p=0.03) but not the NPI (p=0.16) patients had significantly smaller volumes in relation to the normal comparison subjects. NPNN patients had smaller gray matter volumes than did the NPI patients in orbitofrontal, subgenual, and temporal cortices, but these differences were not statistically significant.
Group Differences in the Hippocampus
The effect of group was highly significant (F=7.5, df=2, 94, p=0.0009). NPI patients had significantly smaller hippocampal volumes than did healthy subjects (p=0.0002), whereas the differences between NPNN patients and healthy comparison subjects only approached significance (p=0.07).
Group Differences in the Thalamus
The effect of group was significant (F=3.8, df=2, 62, p= 0.03). The difference between NPI patients and healthy subjects was significant (p=0.008), whereas that between NPNN patients and healthy subjects was not (p=0.23).
Group Differences in the Amygdala
The effect of group was not significant (F=2.3, df=2, 94, p=0.11). The difference between NPNN patients and healthy subjects was significant (p=0.04), however, whereas that between NPI patients and healthy subjects was not (p=0.12).
Group Differences in the Cerebellum
Groups did not differ significantly in cerebellar volumes.
Discriminant Function Analyses
The primary differences between the two patient groups were in volumes of white matter and of the lateral ventricles. The eight white matter volumes significantly discriminated the two patient groups (F=2.08, df=8, 67, p= 0.05), as did the four volumes of the lateral ventricles (F= 2.80, df=4, 71, p=0.03).
Discussion
NPNN patients had markedly less gray matter volume throughout the cerebrum and markedly larger third ventricles than healthy comparison subjects despite having relatively normal cognitive abilities. In several regions, gray matter volumes were actually smaller in the NPNN than in the NPI patients. The magnitude of the gray matter differences was striking and unexpected, given the absent or modest cognitive deficits. Clearly, these patients have highly significant abnormalities in brain structure despite their relatively intact cognition.
NPI patients also had smaller gray matter volumes and larger third ventricles than healthy comparison subjects, with values comparable to those in the NPNN patients. In addition, however, they also had markedly smaller white matter volumes and larger volumes of their lateral ventricles. In other words, differences in abnormality of brain structure between the NPI and NPNN groups appear to be qualitative and not simply quantitative. Gray matter volumes were at least as low in the NPNN patients as in the NPI patients, but the NPI patients had marked abnormalities in volumes of the white matter and lateral ventricles that were not present in the NPNN patients. Indeed, regional volumes in these tissues in NPNN patients did not differ significantly from those in the healthy comparison subjects.
The relatively normal white matter volumes in the NPNN patients and their marked abnormality in the NPI group suggest that white matter pathology may play a primary role in the cognitive deficits observed in most patients with schizophrenia. This is consistent with the view that the cognitive deficits derive from a faulty anatomical configuration and disturbed functioning of neurocognitive systems, rather than from an anatomically localized lesion or set of localized lesions. Some investigators have pointed specifically to the possibility that a faulty integration of cortical-cerebellar-thalamic-cortical circuits may contribute to the cognitive deficits in persons with schizophrenia (22, 23), a disturbance that would likely involve disordered anatomical connections across broad expanses of white matter. Reduced white matter volumes are also often detected in the dementias, where cognitive deficits are a defining feature. Loss of oligodendrocytes seems to be an important contributor to reduced white matter volumes in Alzheimer's disease (24), and indeed animal studies demonstrate that glutamatergic stimulation of N- methyl-d-aspartic acid (NMDA) receptors on the surface of oligodendrocytes affects the development of white matter (25). Thus, either under- or overstimulation of NMDA receptors on oligodendrocytes, especially during childhood or adolescence when myelin is being formed, could produce the abnormalities observed in the NPI subgroup of patients with schizophrenia. Other studies have shown that infections in utero can produce reductions in volume of both white matter and the hippocampus (26). These perinatal and early developmental factors might be particularly relevant in NPI patients, a hypothesis that is amenable to empirical testing. NPI patients were also characterized by markedly larger cerebral ventricles, consistent with previous reports linking increased ventricular size with cognitive deficits (27, 28).
The tests used to define the NPNN and NPI groups were tests of working memory and language-related cognitive operations, two aspects of cognition shown repeatedly to be abnormal in patients with schizophrenia. In previous studies, we have shown the specific tests used to be highly effective in separating patients with schizophrenia from healthy subjects (13). In this study, the NPNN patients performed as well as the healthy comparison subjects on a continuous performance test of sustained attention and showed only a tendency for a difference from healthy comparison subjects on the California Verbal Learning Test of verbal memory. In contrast, NPI patients showed robust deficits on both tests. This demonstrates a general correspondence between subgroup definitions based on the tests used in this study and other more commonly used measures of cognition. We have compared performance on the tests used in this study to performance on a full battery of neuropsychological tests in another sample of 64 patients with schizophrenia or schizoaffective disorder. Correlations between the overall performance score used for group classification and scores on the other tests ranged from 0.35 to 0.54, with the highest correlation being with the full-scale IQ. Defining NPNN patients as within 0.5 standard deviation of the mean of healthy comparison subject, and NPI patients as at least 1.5 standard deviations below the normal mean, there was a 75% correspondence of subgroup assignments based on the full-scale IQ and those based on the overall score on the serial position tests used in the present study. We do not have brain structural data on these patients and so do not know which neuropsychological tests yield greater brain structure differences between groups.
Future work is needed to confirm the main findings of this study that patients with relatively intact cognition have very substantially lower than normal gray matter volumes and that patients with marked cognitive deficits have markedly lower white and gray matter volumes. If confirmed, additional work will be needed to identify the neuropsychological tests that best identify patients with the different patterns of brain structure abnormalities.
Supplementary Material
Acknowledgments
Supported by NIMH grants KO2 MH-01296 and RO1 MH-56642 to Dr. Wexler and NIMH grants MH-068318 and K02 MH-74677 to Dr. Peterson.
Footnotes
The authors report no competing interests.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Bryson GJ, Silverstein ML, Nathan A, Stephen L. Differential rate of neuropsychological dysfunction in psychiatric disorders: comparison between Halstead-Reitan and Luria-Nebraska batteries. Percept Mot Skills. 1993;76:305–306. [PubMed] [Google Scholar]
- 3.Torrey EF, Bowler AE, Taylor EH, Gottesman II. Schizophrenia and Manic-Depressive Disorder: the Biological Roots of Mental Illness as Revealed by the Landmark Study of Identical Twins. Basic Books; New York: 1994. [Google Scholar]
- 4.Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV. Is it possible to be schizophrenic yet neuro-psychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 5.Seaton BE, Allen DN, Goldstein G, Kelley ME, van Kammen DP. Relations between cognitive and symptom profile heterogeneity in schizophrenia. J Nerv Ment Dis. 1999;187:414–419. doi: 10.1097/00005053-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Weickert TW, Goldberg TE, Gold JM, Llewellen BB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 7.Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychol. 2000;109:743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- 8.Rund BR, Sundet K, Asbjornsen A, Egeland J, Landro NI, Lund A, Roness A, Stordal KI, Hugdahl K. Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta Psychiatrica Scandinavica. 2006;113:350–359. doi: 10.1111/j.1600-0447.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 9.Leung WW, Bowie CR, Harvey PD. Functional implications of neuropsychological normality and symptom remission in schizophrenia: a cross-sectional study. J Int Neuropsychol Soc. 2008;14:479–488. doi: 10.1017/S1355617708080600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilk CM, Gold JM, McMahon RP, Humber K, Iannone VN, Buchanan RW. No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 2005;19:778–786. doi: 10.1037/0894-4105.19.6.778. [DOI] [PubMed] [Google Scholar]
- 11.Kay SR, Fiszbein A, Opler L. The Positive and Negative Symptom Scale for Schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 12.Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS. Word and tone working memory deficits in schizophrenia. Arch Gen Psychiatry. 1998;55:1093–1096. doi: 10.1001/archpsyc.55.12.1093. [DOI] [PubMed] [Google Scholar]
- 13.Wexler BE, Jacob S, Stevens AA, Donegan NH. Deficits in language-mediated mental operations in patients with schizophrenia. Schizophr Res. 2002;53:171–179. doi: 10.1016/s0920-9964(01)00194-3. [DOI] [PubMed] [Google Scholar]
- 14.Bruder GE, Wexler BE, Sage MM, Gil RB, Gorman JM. Verbal memory in schizophrenia: additional evidence of subtypes having different cognitive deficits. Schizophr Res. 2004;68:137–147. doi: 10.1016/S0920-9964(03)00156-7. [DOI] [PubMed] [Google Scholar]
- 15.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological Deficits in Neuroleptic Naive Patients With First-Episode Schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 16.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Research Edition. Psychological Corporation; New York: 1987. [Google Scholar]
- 17.Nuechterlein KH. Vigilance in schizophrenia and related disorders. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of Schizophrenia, vol. 5: Neuropsychology, psychophysiology, and information processing. Elsevier; Amsterdam: pp. 397–433. [Google Scholar]
- 18.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in pre-term infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 19.Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen D, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 20.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res Neuroimaging. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 21.Watson C, Andermann F, Gloor P, Jones-Goteman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurol. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 23.Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional disconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjobeck M, Englund E. Glial levels determine severity of white matter disease in Alzheimer's disease: a neuropathological study of glial changes. Neuropathology Appl Neurobiol. 2003;29:159–169. doi: 10.1046/j.1365-2990.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 25.Kolker S, Mayatepek E, Hoffmann GF. White matter disease in cerebral organic acid disorders: clinical implications and suggested pathomechanisms. Neuropediatrics. 2002;33:225–231. doi: 10.1055/s-2002-36741. [DOI] [PubMed] [Google Scholar]
- 26.Debillon T, Gras-Leguen C, Leroy S, Caillon J, Roze JC, Gressens P. Patterns of cerebral inflammatory response in a rabbit model of intrauterine infection-mediated brain lesion. Dev Brain Res. 2003;145:39–48. doi: 10.1016/s0165-3806(03)00193-7. [DOI] [PubMed] [Google Scholar]
- 27.Goetz KL, van Kammen DP. Computerized axial tomography scans and subtypes of schizophrenia: a review of the literature. J Nerv Ment Dis. 1986;174:31–41. doi: 10.1097/00005053-198601000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Kemali D, Maj M, Galderisi S, Salvati A, Starace F, Valente A, Pirozzi R. Clinical, biological, and neuropsychological features associated with lateral ventricular enlargement in DSM-III schizophrenic disorder. Psychiatry Res. 1987;21:137–149. doi: 10.1016/0165-1781(87)90071-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


