Abstract
Study Objectives:
Current sleep scoring rules exclude leg movements that occur near respiratory events from being scored as periodic leg movements during sleep (PLMS) but differ in whether they exclude leg movements occurring at the end (WASM/IRLSSG) or during a respiratory event (AASM). The aim of the present study was to describe the distribution of leg movements in relation to respiratory events and to contribute to an evidence-based rule for the identification and scoring of respiratory-related leg movements (RRLMs).
Design:
Retrospective chart review and analysis of polysomnographic recordings.
Setting:
Clinical sleep laboratory.
Participants:
64 patients with polysomnographic recordings between January 2010 and July 2011, aged 18 to 75 years, with AHI > 20, ODI > 10, more than 50% of apneas being obstructive, > 15 leg movements of any type per hour of sleep, no more than 20% of total sleep time with artifacts and no medical condition or medication that could influence leg movements or respiratory disturbances.
Interventions:
None.
Measurements and Results:
Back-averaging of leg movement activity (LMA) with respect to respiratory events revealed that LMA was present shortly before the end of the respiratory events, but occurred mostly following respiratory events with peak onset of LMA 2.5 s after respiratory event termination. Increased LMA before the beginning of the respiratory event consisted mainly of the tail of LMA after the end of the previous respiratory event. Change-point analysis indicated that LMA was increased over an interval of −2.0 s to +10.25 s around the end of respiratory events. Changing the definition of RRLMs had a significant influence on PLMS counts. The number of patients with obstructive sleep apnea with PLMS index > 15 was 80% when considering the WASM/IRLSSG definition, 67% for the AASM criteria, and 41% when based on the interval identified by change-point analysis (−2.0 to 10.25 s).
Conclusions:
Leg movements are not augmented at the beginning or middle of respiratory events but are increased around the end of respiratory events over a period significantly longer than specified in the AASM and the WASM/IRLSSG rules. Both rules underestimate the number of respiratory-related leg movements and thus overestimate the number of periodic leg movements during sleep in patients with obstructive sleep apnea.
Citation:
Manconi M, Zavalko I, Fanfulla F, Winkelman JW, Fulda S. An evidence-based recommendation for a new definition of respiratory-related leg movements. SLEEP 2015;38(2):295–304.
Keywords: respiratory-related leg movements, periodic leg movements, sleep apnea
INTRODUCTION
Periodic leg movements during sleep (PLMS) are repetitive contractions of the tibialis anterior muscles occurring mainly during NREM sleep. Polysomnographic recording of leg movements is a standard procedure in accredited sleep labs.1 PLMS are observed in many patients with sleep disorders and are common with older age.2 Indeed, an incidental finding of PLMS in the context of sleep related or neurological disorders is frequent in clinical practice.2–5
PLMS are particularly frequent in patients with obstructive sleep apnea (OSA).6–8 However, the role of PLMS in OSA is still unclear due to inconsistent findings concerning the relationship of PLMS to the severity of OSA9 or to sleepiness in OSA patients.6,7,10,11 In addition, during treatment of OSA with continuous positive airway pressure (CPAP), both a significant decrease and a new emergence of PLMS have been observed.8,12–16 Therefore, the presence of PLMS during CPAP treatment has been interpreted both as indicating the persistence of sleep disordered breathing14,16 or alternatively, as sufficient treatment of breathing disorders “unmasking” preexisting PLMS.8,13 The assessment of PLMS in the presence of sleep-related breathing disorders is complicated by the concern that leg movements may be triggered by the respiratory events and are not part of a PLM sequence.17 Indeed, the two major international scoring guidelines for PLMS specifically exclude respiratory-related leg movements (RRLMs) from the scoring of PLMS.1,17
The two scoring guidelines differ, however, significantly in their definition of RRLMs. The criteria established by a task force of the International RLS Study Group (IRLSSG) and endorsed by the World Association of Sleep Medicine (WASM) consider leg movements as respiratory-related when a leg movement occurs at the end (± 0.5 s) of an apnea or hypopnea.17 In contrast, the 2007 scoring manual of the American Academy of Sleep Medicine (AASM) defines RRLMs as leg movements that should not be scored if they occur “during a period from 0.5 seconds preceding an apnea or hypopnea to 0.5 seconds following an apnea or hypopnea.”1 This definition has recently been extended to include also respiratory effort related arousals and other sleep disordered breathing events.13 To the best of our knowledge, neither of the criteria to identify and quantify RRLMs were evidence-based, and they have not been assessed using the recent computerized approaches.18 In particular, the real time-structure of RRLMs, i.e., the distribution of leg movements (LMs) with respect to the breathing events, has never been systematically analyzed.
The overall aim of the present study was to depict the time structure of RRLM to inform an evidence-based scoring approach. To this end, we investigated the distribution of LMs in relation to apneas and hypopneas in a sample of patients with OSA. Since the major difference between the WASM/IRLSSG and AASM criteria is the consideration of leg movements during and before a respiratory event, we explored whether LMs are specifically increased during, at the onset of, or following respiratory events. Furthermore, we sought to derive empirically based estimates for the interval during which LMs are increased in relation to apneas and hypopneas. Finally, we illustrate the effect of changing the criteria for RRLMS on the scoring of PLMS.
METHODS
Subjects
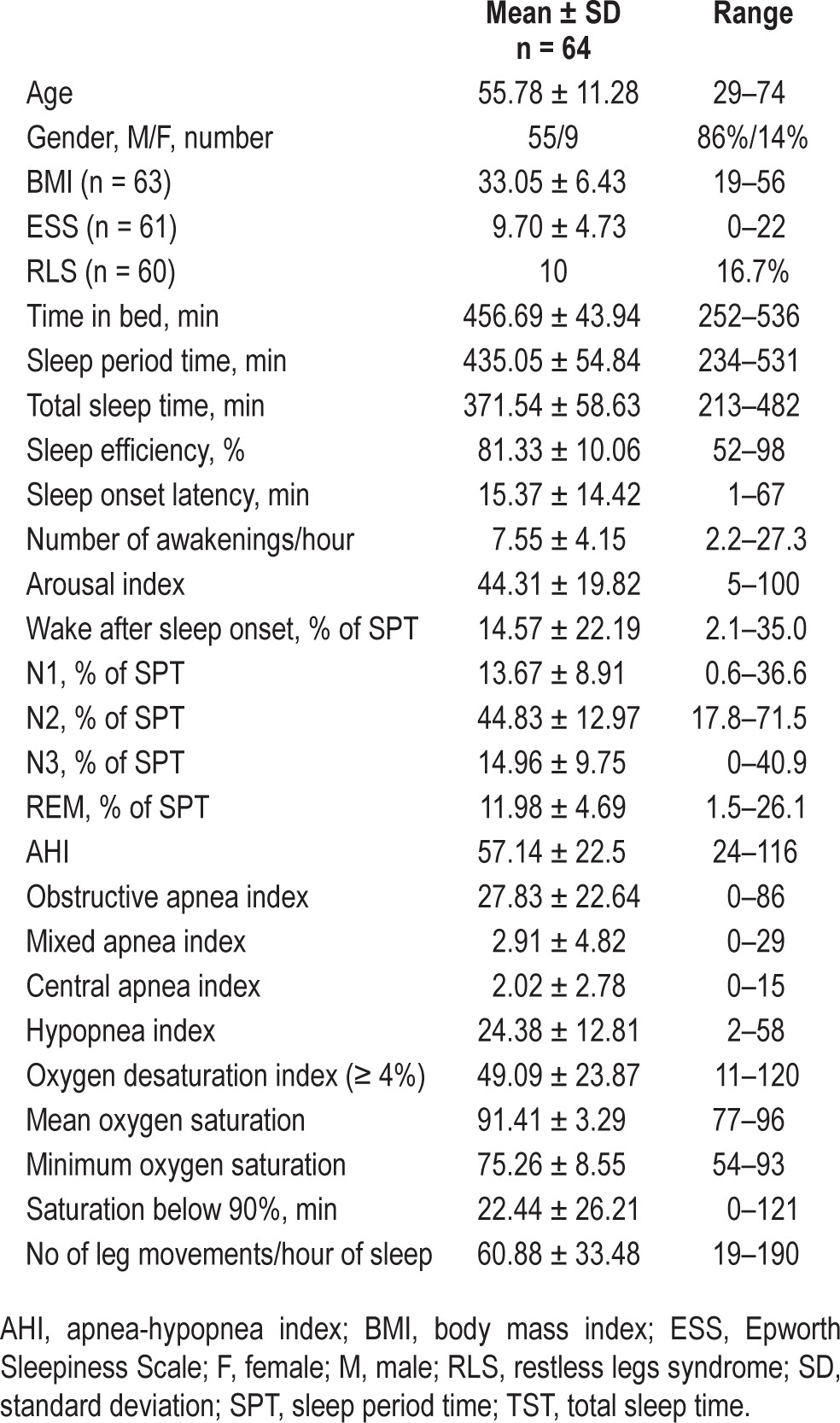
We considered all patients with full video-polysomnographic (PSG) recordings between January 2010 and July 2011 (n = 682), with an apnea-hypopnea index (AHI) > 20, an oxygen desaturation index (ODI, 4%) > 10, and more than half of all observed apneas scored as obstructive (n = 178). Patients had to be between 18 and 75 years (n = 163), without any medical condition or medication that could affect sleep-related leg movements or respiration (n = 94). In addition, > 80% of total sleep time had to be free of artifacts (n = 84). From these, we selected patients with > 15 LMs of any type per hour of sleep (n = 64). Of these 64 patients, 55 were male and 9 female with an average age of 55.8 years (SD: ± 11.3 years, Table 1). The study was approved by the local ethics committee.
Table 1.
Description of participants.
Sleep Recordings
Nocturnal PSGs were carried out in a standard sound-attenuated (noise level to a maximum of 30 dB nHL) sleep laboratory room. All PSG recordings included 6 monopolar EEG channels (F3/M2, F4/M1, C3/M2, C4/M1, O1/M2, O2/M1), submental electromyogram, electrooculogram, electrocardiogram, respiratory parameters (oro-nasal airflow pressure cannula, thoracic and abdominal effort strain gauge, pulse oximetry), and electro-myogram of the right and left tibialis anterior muscles (bipolar derivations with two electrodes placed 2 cm apart on the anterior tibialis muscle of each leg); impedance was kept less < 10 KΩ, according to the AASM criteria.1 All PSGs had been recorded and scored according to the 2007 rules of the American Academy of Sleep Medicine.1
Scoring of Respiratory Events and Leg Movements
For the current study, in all PSG recordings all LMs and respiratory events (apneas and hypopneas) were manually re-scored by a single sleep expert (I.Z.) according to the following AASM criteria1:
Hypopnea: A reduction ≥ 50% in nasal airflow pressure signal excursion for ≥ 10 s, associated with either ≥ 3% desaturation or an arousal.
Apnea: A reduction ≥ 90% in nasal airflow pressure signal excursion for ≥ 10 s.
The onset of the hypopnea or apnea was defined as the nadir of the flow signal preceding the first breath that was clearly reduced. The end of the respiratory event was defined as the beginning of the first breath that approximated the baseline breathing amplitude. The type of apnea (central, obstructive, or mixed) was determined according to AASM criteria.1
Leg movements were scored according to AASM19 and WASM/IRLSSG17 criteria as any leg EMG increase ≥ 8 μV above the resting baseline and lasting between 0.5 and 10 s. The onset of the LM was defined as the beginning of the EMG increase ≥ 8 μV above the resting baseline, and the end of the LM was defined as the beginning of the period where the EMG decreases for at least 0.5 s to < 2 μV above resting baseline.
Leg movements were scored as respiratory-related according to both AASM19 and WASM/IRLSSG17 criteria:
Respiratory-related leg movements according to AASM criteria: Any leg movement that occurred in the interval from 0.5 s before the start of an apnea or hypopnea to 0.5 s after the end of the apnea or hypopnea.
Respiratory-related leg movements according to WASM/ IRLSSG criteria: Any leg movement that occurred in the interval from 0.5 s before the end of an apnea or hypopnea to 0.5 s after the end of the apnea or hypopnea.
Procedure
Demographic and anthropometric information was extracted from the patients' medical records. From each recording, we extracted sleep stage information and the onset (in milliseconds) and duration of each individual LM and respiratory event. The total number of LMs therefore contained isolated LMs, PLMS, and RRLMs. All events where artifacts precluded the determination of the exact time of onset and duration were excluded from all analyses. In addition, we selected only LMs and respiratory events with the beginning, middle, and end during an epoch classified as sleep and with none of these epochs bordering on wake, i.e., the epoch before the epoch during which the event started and the epoch after the epoch during which the event ended had to be classified as sleep.
Both the AASM and the WASM/IRLSSG consider LMs as respiratory-related when any part of the LM lies within the spec-ified interval, i.e., they refer to leg movement activity (LMA) rather than leg movement onsets. We therefore converted LMs to LMA as described below.
Descriptive and Statistical Analyses
Distribution of Respiratory-Related Leg Movements
In a first step we explored the distribution of LMA in relation to respiratory events. Because respiratory events and inter-respiratory intervals, i.e., the interval between the end of one respiratory event and the onset of the subsequent event, varied widely in duration, all LMA was back-averaged separately to the onset, the middle, and the end of the respiratory events. Back-averaging of LMA consisted of the following steps:
(1) For each respiratory event and event time point (onset, middle, end) we selected the interval from 30 s before to 30 s after the event.
(2) This interval of 60 s was divided into 0.25-s bins, resulting in a segment with 240 bins for each event.
(3) Based on the information (in ms) about onset and duration of all LMs during this interval, each 0.25-s bin was classified as containing or not-containing any part of a LM, denoted with 1 or 0, respectively.
(4) Finally, the single interval segments were summed for each event and all subjects. The result is a summary segment that is aligned to the same event (onset, middle, or end of respiratory events) and that describes the number of LMs observed for each 0.25-s bin in the interval of ± 30 s around the event.
This describes the absolute distribution of LMA in relation to respiratory events. We separately depicted the absolute distribution of LMA that were classified as RRLMs according to the WASM/IRLSSG rules, the AASM criteria, and all LMs in the interval of ± 30 s around the onset, middle, and end of the respiratory events.
Leg Movements during Respiratory Events
Next, we investigated whether there was any evidence that LMs were increased during a respiratory event compared to the onset or end of the events. To distinguish LMs during a respiratory event from LMs at the onset and end of events, we back-averaged LMA to the middle of respiratory events of different durations. In addition, we constructed the relative distribution of LMs by setting the respiratory event duration to 100%, where 0% is the onset of the event, 50% the middle, and 100% is the end of the event. The occurrence of LMA was then transformed to this common relative metric. This allowed us to graph the complete distribution of LMA over the whole duration of the respiratory event.
Leg Movements at the Onset and End of Respiratory Events
We then addressed the question whether LMA is increased both before the onset of a respiratory event and at the end of it. In our sample the inter-respiratory event intervals, i.e., the time from the end of one respiratory event to the beginning of the next one, was typically between 15 s and 20 s. Since the above analyses suggested that LMA is increased roughly from 20 s before the onset of an event and up to 15 s after the end of an event, this posed the question whether LMA before the start of the respiratory event is just the LMA belonging to the end of the previous respiratory event and vice versa. For these analyses we considered only the nearest respiratory event, i.e., each LM was associated to only one, the nearest, end and/or beginning of a respiratory event. We therefore explored:
the proportion of LMs that occurred (i) only around the end of respiratory events, (ii) only at the onset of events, or (iii) in both intervals. The intervals that were considered were from −10 s to +30 s around the end and −30 s to +10 s around the onset of the respiratory events;
the relative distribution of LMs in the inter-respiratory event intervals. For this analysis, the distribution was standardized to the inter-respiratory event duration with 0% being the end of a respiratory event and 100% the onset of the subsequent event.
Identifying Intervals with Increased Leg Movements
Next, we sought to derive empirically based estimates for the interval during which LMA is increased in relation to apneas and hypopneas. While the graphical representation of the distribution of LMA in relation to respiratory events can roughly identify intervals with increased LMA around the respiratory events, for an empirically based identification an objective statistical criterion is needed. We chose change point analysis to address this question. Change point analysis refers to a set of methods developed for the problem of estimating the points at which the statistical properties of a sequence of observations change.20 Briefly, a change point is a point in an ordered sequence of observations where the statistical properties (e.g., the mean) of the series changes such that the property of the sequence before the change point is significantly different from the property of the sequence after the change point. In the context of the present study, the ordered sequence, or vector, is represented by the number of those with LMA (or the number of LM onsets) in each 0.25-s bin in the interval from −30 s to +30 s around the end of the respiratory events. The number of those with LMA and the number of LM onsets were derived from back-averaging, as described above. Change point analysis then finds the optimal position, i.e., the time point, where the mean of the sequence before the time point is significantly different from the mean of the sequence after the identified time point. In our case, that signifies that the number of those with LMA (or number of LM onsets) before the identified change point is significantly larger or smaller than after the change point. We used binary segmentation change point analysis, which is an approximate method and employs the cumulative sums test statistic to find the optimal positioning and number of change points in the mean for data where no assumption about the distribution is made.21
Effect of Different Criteria for Respiratory-Related Leg Movements on Other Leg Movement Parameters
Finally, we explored the effect of changing the scoring rules for RRLMs. We computed the indices for RRLMs, PLMS, and isolated LMs as well as the proportion of subjects with elevated PLMS (PLMSI > 15) based on 4 different definitions: (i) the WASM/IRLSSG definition; (ii) the AASM definition; and the definition derived from the change point analysis for (iii) LMA and (iv) the onset of LMs. Differences between the definitions were tested with analysis of variance and χ2 tests, as appropriate.
All computations and analyses were performed with R22 and the change point package23 in R.
RESULTS
A total of 64 patients (55 male, 9 female, mean age 56 ± 11 years) were included in the study. The individual AHI varied from 24 to 116 (mean AHI 57 ± 22) and patients contributed between 136 and 706 apneas and hypopneas. The LM index varied between 19 and 190 (mean LMI 61 ± 33), and between 89 and 1,130 LMs were observed for each patient.
Among all patients there were 28,507 LMs during sleep; 398 were excluded because of technical failures and 6,050 because they occurred in epochs that bordered on wake epochs, leaving 22,059 LMs with individual patients contributing between 82 and 965 LMs to the analysis. The analysis included 21,240 apneas or hypopneas (105 to 665 from each patient) after excluding 1,546 events (technical failure: 210, near-wake epochs: 1,978).
Distribution of Leg Movement Activity
According to the WASM/IRLSSG definition, 3,641 LMs (16.55% of all LMs) were respiratory-related, and for 3,641 respiratory events (17.13%) one or more LM occurred within 0.5 sec around the end of the respiratory event. According to the AASM rules, almost twice as many, i.e., 6,916 (31.35%), LMs were classified as respiratory-related and 5,763 respiratory events (27.13%) had one or more LM associated with it.
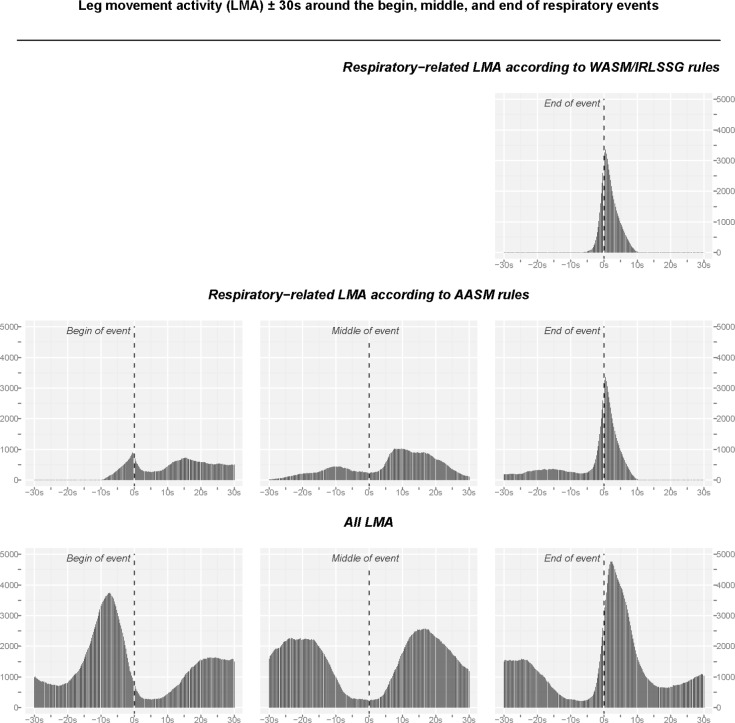
Figure 1 shows the distribution of LMA around the beginning, middle, and end of respiratory events for respiratory-related LMA according to the WASM/IRLSSG rules (upper right panel) and the AASM rules (middle panels). All LMA around the respiratory events is shown in the lower panels. The most salient points that can be derived from these distributions are that, indeed, there is substantially increased LMA around the beginning and end of respiratory events, and that the interval over which LMA is increased is substantially wider than the one assumed for either set of scoring rules.1,17
Figure 1.
Distribution of leg movement activity (LMA) ± 30 s around the beginning, middle, and end of respiratory events. The distribution of LMA was constructed based on the information (in ms) about the onset and duration of LMs that occurred within the depicted interval (for details see Methods). Upper panel: Distribution of respiratory-related LMA according to the WASM/IRLSSG criteria, which consider only the interval ± 0.5 s around the end of the respiratory event. Middle panel: respiratory-related LMA according to the AASM criteria, which consider an interval of −0.5 s before the beginning to +0.5 s after the end of respiratory events. Lower panel: All LMA observed around the beginning, middle, and end of respiratory events.
Distribution of Leg Movement Activity in the Middle of Respiratory Events
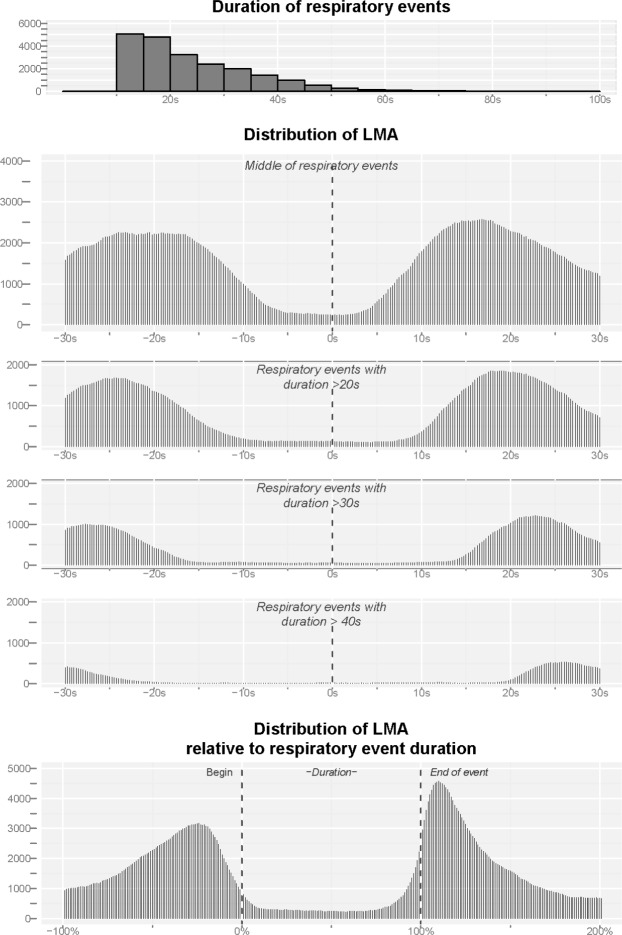
To explore whether LMA is increased during a respiratory event, we graphed LMA around the middle for respiratory events of variable duration (Figure 2). Duration of respiratory events varied from 10 to 90 s with a median duration of 21 s (interquartile range: 15–31; Figure 2, upper panel). Back-averaging LMA to the middle of respiratory events showed no indication that LMA is increased during a respiratory event and that the peaks in LMA before and after the middle of the event are strictly related to the beginning and end of the respiratory event itself (Figure 2, middle panels). This is further supported by the relative distribution of LMA (Figure 2, lower panel) that does not show an increase of LMA during respiratory events.
Figure 2.
Upper panel: Distribution of the duration of respiratory events. Middle panels: Distribution of leg movement activity (LMA) around the middle of respiratory events, for all events and events with a minimum duration of 20 s, 30 s, and 40 s. The distribution of LMA was constructed based on the information (in ms) about the onset and duration of LMs that occurred within the depicted interval. Lower panel: Distribution of all LMA relative to the duration of the respiratory event. In addition, we constructed the relative distribution of LMs was obtained by setting the respiratory event duration to 100%, where 0% is the onset of the event, 50% the middle, and 100% is the end of the event. The occurrence of LMA was then transformed to this common relative metric.
Distribution of Leg Movement Activity at the Beginning vs. the End of Respiratory Events
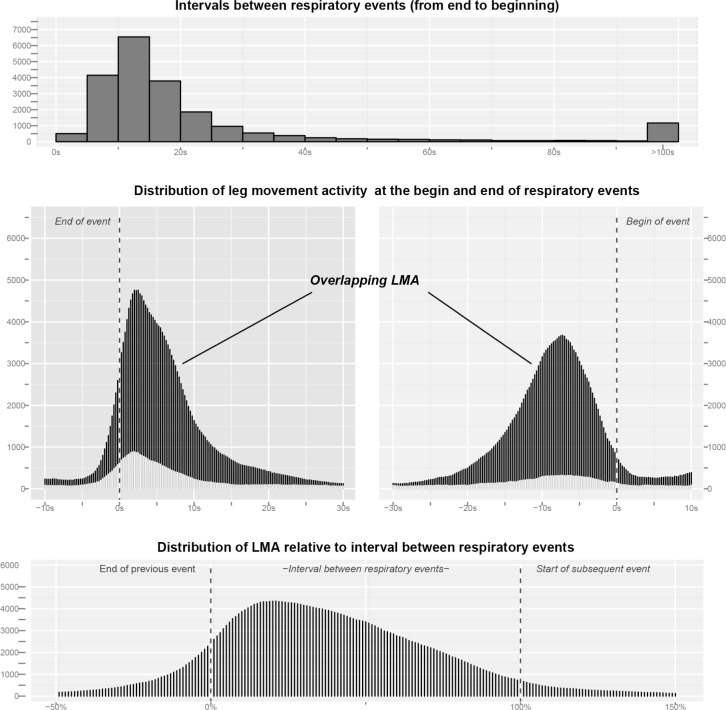
In our sample, the most typical inter-respiratory-event interval was 15 s to 20 s (median 15 s, interquartile range 10 s to 22 s; Figure 3, upper panel) and therefore the majority of LMs until 15 s after the end of a respiratory event will be at the same time within 20 s before the start of the next event. Considering the very broad ranges from −10 s to +30 s around the end and −30 s to +10 s around the beginning of the respiratory events, 67.6% (14,906) of all LMs occurred at the end, 63.2% (13,944) at the beginning, and 77.2% (17,024) in either one and/or the other interval (Figure 3, middle panels). A total of 11,826 LMs were contained in both intervals at the same time, which represents 79.3% of all LMs around the end and 84.8% of all LMs around the beginning.
Figure 3.
Upper panel: Distribution of the intervals between respiratory events measured from the end of the first respiratory event to the beginning of the subsequent respiratory event. Middle panel: Distribution of leg movement activity (LMA) around the end (−10 s to +30 s, left middle panel) and the beginning of respiratory events (−30 s to +10 s, right middle panel). The dark parts refer to overlapping LMA, i.e., leg movements that occurred in both intervals, the light gray parts refer to remaining, unique leg movements not contained in the other interval. Of all LMs, 77.2% occurred in either one or both of these intervals. Of these, 69.5% occurred in both intervals, which represents 79.3% of all LMs around the end and 84.8% of all LMs around the onset of respiratory events. Lower panel: Distribution of LMA relative to the duration of the interval between respiratory events. Here, 0% denotes the end of the first event and 100% the start of the subsequent event.
Since most of the LMs occurred between respiratory events, we standardized LMA to the inter-respiratory event duration with results given in the lower panel of Figure 3. The relative distribution shows a single peak only, which is shifted towards the left, i.e., the end of the respiratory event.
Respiratory-Related Leg Movements: Evidence to Support a Modification of the Rules
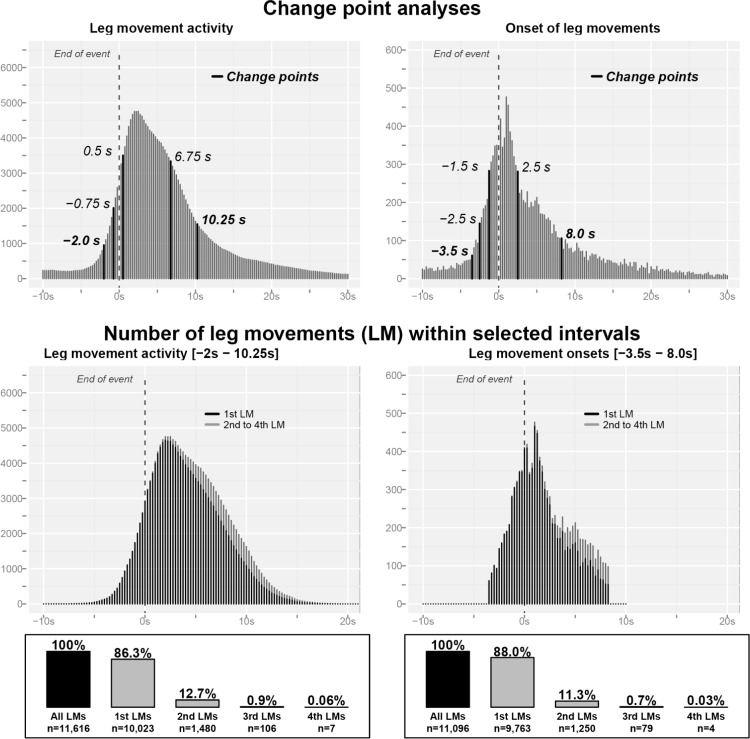
Next, we sought to derive empirically based estimates for the interval during which LMs are increased in relation to apneas or hypopneas. As detailed in the method section, we used change point analysis to identify those points where the distribution of LMA or LM onsets changed. The results are illustrated in Figure 4 and show that change point analysis identified 5 change points both for LMA and LM onsets. Considering the first change point as the point where LMs significantly increased for the first time, and the last change point as the one after which no further change was observed, this analysis suggests that LMA is increased over an interval of −2.0 s to +10.25 s around the end of respiratory events. The corresponding interval is −3.5 s to +8.0 s for LM onsets. Both intervals contain a roughly similar number of LMs: 11,616 (52.7% of all LMs) and 11,096 (50.3%) for LMA and LM onset, respectively.
Figure 4.
Upper panel: Results of the change point analyses (see Methods). Change point analyses identified time points where a significant change in the statistical properties of the sequence occurs. The analyses identified 5 change points both for leg movement activity (LMA, left panel) and the onsets of leg movements (right panels). Middle panel: Distribution of the number of leg movements within the intervals identified by change point analysis. The dark parts show the distribution of the first leg movement in this interval, the light gray parts show the distribution of the 2nd to 4th leg movements during that interval. Lower panel: Frequency of multiple leg movements in the identified intervals. In the vast majority of cases (∼87%) only one leg movement was observed around the end of respiratory events.
Because both intervals are considerably longer than previously assumed, we investigated how many LMs were contained in this interval to explore the question whether the wideness of the intervals was due not only to an increase of the occurrence of LMs but also because of an increased number of LMs linked to a single breathing event. The results are given in the lower panel of Figure 4 and show that this was not the case. In 86.3% and 88.0% of the cases, the intervals contained only a single LM based on the above identified cutoff values for LMA and LM onset, respectively. Around 12% of the intervals contained a second LM, but 3 or a maximum of 4 LMs was a very rare occurrence. Although these 2nd to 4th LMs occurred preferentially in the second half of the intervals (Figure 4), they were rather evenly distributed over this part of the interval and did not seem to change the basic distribution of LMs.
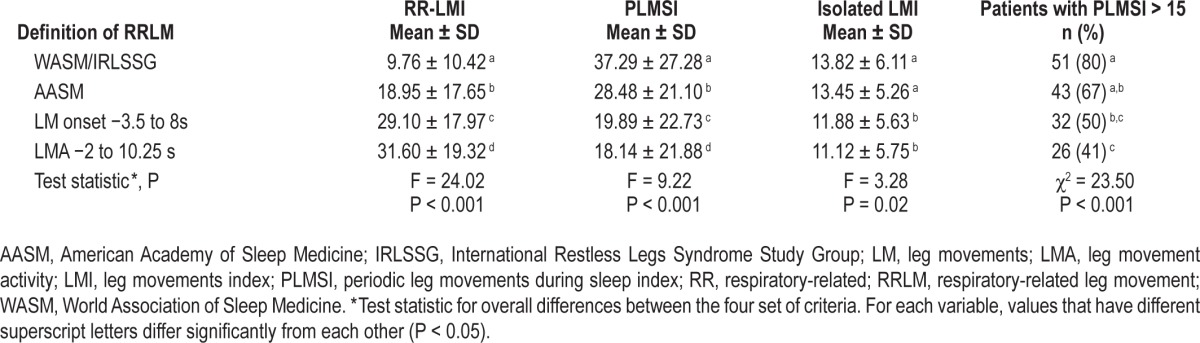
Influence of Scoring Rules on Respiratory-Related and Periodic Leg Movements
To illustrate the effect of changing the scoring rules for RRLMs, we computed the indices for RRLMs, PLMS, and isolated LMs based on the 4 different definitions (WASM/ IRLSSG, AASM, interval based on LM onset and LMA). The results are given in Table 2. As was to be expected, the RRLM index was lowest for the WASM/IRLSSG definition, considerably larger for the AASM definition, and even larger for our definitions based on LM onset and LMA. Switching from the WASM/IRLSSG definition to one that is based on LMA almost halved the number of patients with increased PLMS (PLMS index > 15) from 80% to 41% (Table 2).
Table 2.
Leg movement indices for different definitions of respiratory-related leg movements.
DISCUSSION
The present study described the temporal distribution of LMs in relation to respiratory events in patients with moderate to severe OSA. To the best of our knowledge, this is the first comprehensive study of RRLM temporal distribution. As such, this study yielded several new findings with potential implications for clinical sleep medicine and research.
We could show that LMs are increased at the end and also at the beginning of respiratory events. The increase in LMs started shortly before the end of the respiratory event, peaked around 2 s after the breathing event, and lasted until 8 to 10 s after the end of the event, depending on whether the onset of LM or LMA was considered. LMs were also increased before the beginning of an event, but our results argue that in the majority of cases LMs occurring during this inter-apnea interval are more closely associated with the end of the previous respiratory event.
We also found no indication that LMs are specifically increased in the middle of respiratory events. However, we cannot exclude that the low number of LMs during a respiratory event is not indeed higher than would be observed during apnea-free periods. Nevertheless, compared to the prominent increase at the end of events, these seemed negligible. Thus, our results support a rule that does not include LMs at the beginning or middle of respiratory events.
In addition, the empirical distribution of LMs clearly suggested that the interval for determining respiratory-related LMs is too narrow in both sets of rules and neglects the majority of LMs associated with respiratory events. Specifically, the cutoff value of 0.5 s after the end of a respiratory event misses the strong increase in LMs, which peaks around 2 s. This suggests that the temporal cutoff values to identify RRLMs at the end of breathing events should be enlarged. Our analysis identified an interval from −2 to +10.25 s or from −3.5 to 8 s depending on whether LMA or just the onset of LMs is considered, respectively. Concerning this last point, it remains open whether it is more appropriate to consider only the onset of LMs or LMA. Although our study did not solve this issue, it describes the detailed pictures of the two scenarios, demonstrating that the impact of one or the other choice on the final outcome (LM computation) is less relevant than expected (Figure 4). A similar argument holds true for the issue of whether it is more appropriate to consider all LMs occurring within the mentioned cutoff values around the end of respiratory events or just the first LM. Even here the issue remains open but may indeed have less relevance, since we demonstrated that in less than 1% of the cases there are more than 2 LMs associated to the end of a respiratory event, and in more than 86% of the cases only a single LM is observed (Figure 4).
Finally, changing the rules for RRLM directly affected the scoring of PLMS. PLMS counts were highest when considering the WASM/IRLSSG definition, moderately lower when considering the AASM definition, and decidedly lower when based on the interval identified by change point analysis. The definition of RRLMs therefore has a substantial effect on clinical decision making in patients with OSA. Importantly, our results suggest that changing the rules will have a direct effect on the number of PLMS as well as on the number of RRLMs, rather than on isolated ones. The immediate implication of this is that by using the existing rules, PLMS in patients with obstructive sleep apnea may have been considerably overestimated, while the number of RRLMs is considerably underestimated.
These results will have to be confirmed in other independent studies. If they are confirmed, the past literature concerning PLMS and breathing disorders needs to be carefully reevaluated. Among several issues, the possible impact of these new findings might concern such important topics as the still-controversial role of LMs in OSA patients with residual somnolence after CPAP treatment,6,11 the impact of RRLMs on the cardiovascular system,6,24 the prognostic values of RRLMs/PLMS in special pathological conditions such as heart failure25,26 or renal failure,27–29 the impact of RRLMs on sleepiness independent of the severity of sleep apnea,7 and, in general all the possible speculation postulated around the meaning of this specific motor activity.7
Our study has several limitations, which have to be taken into account when interpreting the results. For one, we selected patients with moderate to severe OSA, and it is unclear whether our results also hold for patients with mild OSA and central sleep apnea. Moreover, we only considered apneas and hypopneas and did not include respiratory effort related arousals (RERAs), which have been recently included in the updated AASM scoring rules.19 Furthermore, while there exists a very precise definition for the onset and end of leg movements1,17 determining the end of respiratory events is less well defined by comparison.1 This may have resulted in a reduced precision in determining the onset and end of respiratory events which would also translate into a reduced precision of the timing between LMs and respiratory events. Finally, we did not consider other, possibly relevant, LM features such as the amplitude, duration, and periodicity of RRLM, which could contribute to a better differentiation of LM phenotypes.
At the end, it is imperative to highlight that our study is an objective new picture on the complex landscape of the temporal relationship between LMs and breathing events, without any pretension to investigate the pathogenic nature of RRLMs and their potential different genesis compared to PLMS. By using these results, further investigations are warranted in order to explain the mechanism generating RRLMs and even more important to answer the fundamental question whether it is correct and clinically useful to distinguish PLMS from RRLMs. In conclusion, our results obtained by a systematic analytic approach to RRLMs strongly argue for a revision of the current scoring criteria for RRLMs, which should consider RRLMs only at the end of breathing events and over a wider temporal window, considerably longer than the ones suggested by current rules.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the sleep lab team of the Sleep and Epilepsy Center at the Neurocenter of Southern Switzerland in Lugano and, in particular, Miss Elisabetta Colamartino, for their valuable help in conducting this study.
REFERENCES
- 1.Iber C, Ancoli-Israel S, Chesson A, Quan S American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 2.Ferri R. The time structure of leg movement activity during sleep: the theory behind the practice. Sleep Med. 2012;13:433–41. doi: 10.1016/j.sleep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 5.Dauvilliers Y, Pennestri MH, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res. 2007;16:333–9. doi: 10.1111/j.1365-2869.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:281–7. [PubMed] [Google Scholar]
- 7.Chervin RD. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164:1454–8. doi: 10.1164/ajrccm.164.8.2011062. [DOI] [PubMed] [Google Scholar]
- 8.Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26:717–20. doi: 10.1093/sleep/26.6.717. [DOI] [PubMed] [Google Scholar]
- 9.Warnes H, Dinner DS, Kotagal P, Burgess RC. Periodic limb movements and sleep apnoea. J Sleep Res. 1993;2:38–44. doi: 10.1111/j.1365-2869.1993.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C, Philip P. Tiredness and somnolence despite initial treatment of obstructive sleep apnea syndrome (what to do when an OSAS patient stays hypersomnolent despite treatment) Sleep. 1996;19(9 Suppl):S117–22. doi: 10.1093/sleep/19.suppl_9.s117. [DOI] [PubMed] [Google Scholar]
- 11.Vernet C, Redolfi S, Attali V, et al. Residual sleepiness in obstructive sleep apnoea: phenotype and related symptoms. Eur Respir J. 2011;38:98–105. doi: 10.1183/09031936.00040410. [DOI] [PubMed] [Google Scholar]
- 12.Pai V, Khatwa U, Ramgopal S, Singh K, Fitzgerald R, Kothare SV. Prevalence of pediatric periodic leg movements of sleep after initiation of PAP therapy. Pediatr Pulmonol. 2014;49:252–6. doi: 10.1002/ppul.22802. [DOI] [PubMed] [Google Scholar]
- 13.Hedli LC, Christos P, Krieger AC. Unmasking of periodic limb movements with the resolution of obstructive sleep apnea during continuous positive airway pressure application. J Clin Neurophysiol. 2012;29:339–44. doi: 10.1097/WNP.0b013e3182624567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo WH, Guilleminault C. Periodic leg movement, nasal CPAP, and expiratory muscles. Chest. 2012;142:111–8. doi: 10.1378/chest.11-1563. [DOI] [PubMed] [Google Scholar]
- 15.Fry JM, DiPhillipo MA, Pressman MR. Periodic leg movements in sleep following treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Chest. 1989;96:89–91. doi: 10.1378/chest.96.1.89. [DOI] [PubMed] [Google Scholar]
- 16.Briellmann RS, Mathis J, Bassetti C, Gugger M, Hess CW. Patterns of muscle activity in legs in sleep apnea patients before and during nCPAP therapy. Eur Neurol. 1997;38:113–8. doi: 10.1159/000113173. [DOI] [PubMed] [Google Scholar]
- 17.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Ferri R, Zucconi M, Manconi M, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep. 2005;28:998–1004. doi: 10.1093/sleep/28.8.998. [DOI] [PubMed] [Google Scholar]
- 19.Berry R, Brooks R, Gamaldo C, et al. Darien, IL: American Academy of Sleep Medicine; 2013. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.0.1. www.aasmnet.org. [Google Scholar]
- 20.Chen J, Gupta AK. Boston, MA: Birkhäuser; 2000. Parametric statistical change point analysis. [Google Scholar]
- 21.Scott A, Knott M. A cluster analysis method for grouping means in the analysis of variance. Biometrics. 1974;30:507–12. [Google Scholar]
- 22.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2011. R: a language and environment for statistical computing. [Google Scholar]
- 23.Killick R, Eckley I. Changepoint: an R package for changepoint analysis. R package version 0.8. 2012 [Google Scholar]
- 24.Yang CK, Jordan AS, White DP, Winkelman JW. Heart rate response to respiratory events with or without leg movements. Sleep. 2006;29:553–6. doi: 10.1093/sleep/29.4.553. [DOI] [PubMed] [Google Scholar]
- 25.Skomro R, Silva R, Alves R, Figueiredo A, Lorenzi-Filho G. The prevalence and significance of periodic leg movements during sleep in patients with congestive heart failure. Sleep Breath. 2009;13:43–7. doi: 10.1007/s11325-008-0207-5. [DOI] [PubMed] [Google Scholar]
- 26.Yumino D, Wang H, Floras JS, et al. Relation of periodic leg movements during sleep and mortality in patients with systolic heart failure. Am J Cardiol. 2011;107:447–51. doi: 10.1016/j.amjcard.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 27.Benz RL, Pressman MR, Hovick ET, Peterson DD. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000;35:1052–60. doi: 10.1016/s0272-6386(00)70039-4. [DOI] [PubMed] [Google Scholar]
- 28.Jung HH, Lee J, Baek HJ, Kim SJ, Lee JJ. Nocturnal hypoxemia and periodic limb movement predict mortality in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2010;5:1607–13. doi: 10.2215/CJN.08881209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portaluppi F, Cortelli P, Buonaura GC, Smolensky MH, Fabbian F. Do restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) play a role in nocturnal hypertension and increased cardiovascular risk of renally impaired patients? Chronobiol Int. 2009;26:1206–21. doi: 10.3109/07420520903245276. [DOI] [PubMed] [Google Scholar]