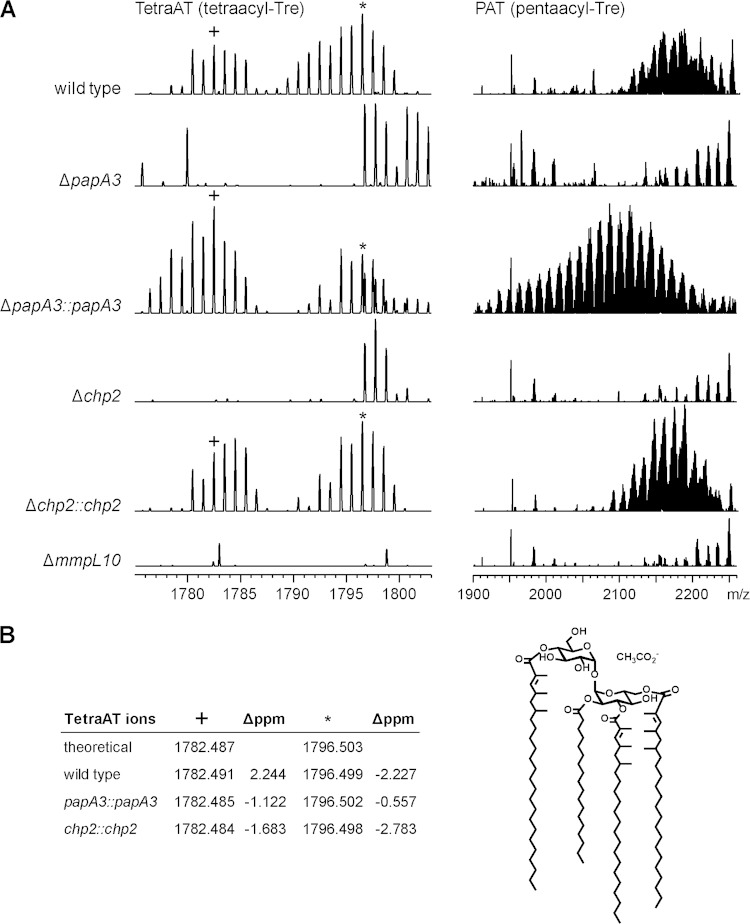
FIG 3.
Chp2 and MmpL10 are required for biosynthesis of PAT and the novel acyltrehalose TetraAT. M. tuberculosis wild-type, ΔpapA3, Δchp2, and ΔmmpL10 strains were analyzed by ESI-MS. (A) TetraAT and PAT were absent from all three mutants but were restored in the ΔpapA3 and Δchp2 complement strains. PAT appears as a characteristic envelope of peaks centered at approximately m/z 2,100 to 2,200. A representative segment of the TetraAT spectrum is shown in order to highlight two of the peaks used for assignment. (B) TetraAT was assigned by exact mass, and observed ions were within 3 ppm of the predicted m/z value. As an example, a possible structure for the m/z 1,782.487 [M+CH3CO2-] ion is shown.