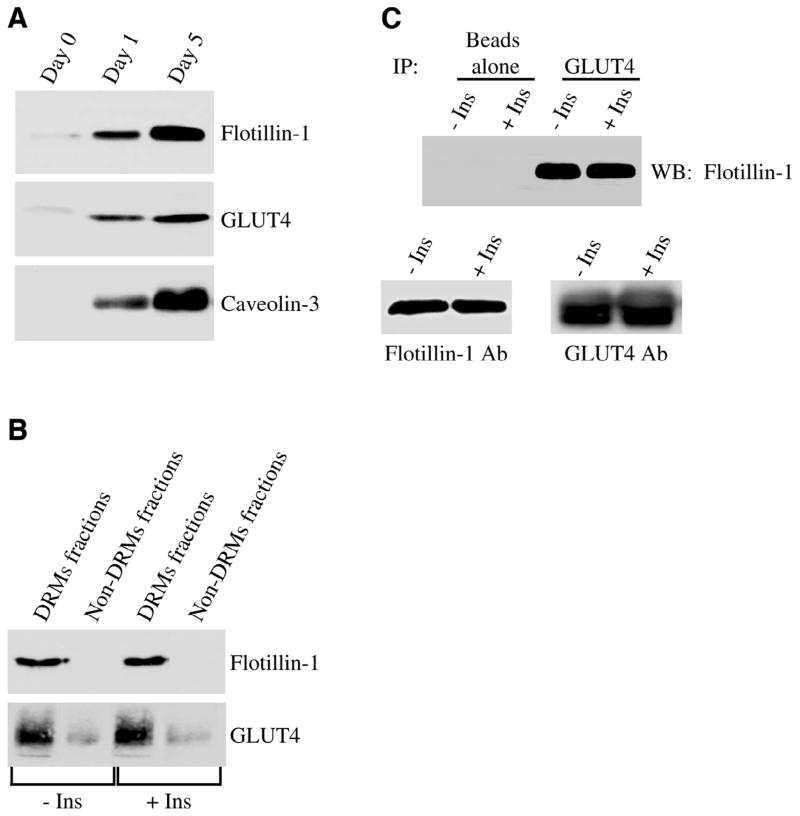
Figure 1.
Flotillin-1 and GLUT4 are part of same protein complex in skeletal muscle cells. A) Immunoblot analysis. Skeletal muscle cells were left undifferentiated or differentiated for different periods of time (1 and 5 days). Cell lysates were then subjected to immunoblot analysis with anti-flotillin-1, anti-GLUT4, and anti-caveolin-3 IgGs. B) Sucrose density gradient centrifugation. Skeletal muscle cells were differentiated for 5 days, treated with or without insulin (160 nM for 10 min), and detergent-resistant microdomains (DRMs fractions) were separated from the bulk of cellular membranes and cytosolic proteins (Non-DRMs fractions) by equilibrium sucrose density gradient centrifugation. Expression of flotillin-1 and GLUT4 was examined by imunoblotting analysis using specific antibody probes. C) Co-immunoprecipitation. Skeletal muscle cells were differentiated for 5 days and treated with or without 160 nM insulin for 10 min. Cell lysates were immunoprecipitated with anti-GLUT4 IgGs. Immunoprecipitates were subjected to Western blotting analysis using an antibody probe specific for flotillin-1. Immunoprecipitation with beads alone was used as an internal control. Insulin did not change either flotillin-1 or GLUT4 total protein expression (lower panels).